PNL Volume 21 1989 RESEARCH
REPORTS
79
Table 2. Influence of X-ray doses on the incidence of altered
RQ
regenerated plants and on the
frequency of somatic mutant sectors in lines 18-1 and
17-35.
|
Dose
(kr) |
Number of regenerated
plants |
Altered plants
(%) |
Frequency of mutant sectors, % |
|
|
||
|
yellow
aa |
dark-green
AA |
twin
AA/aa |
|||||
|
18-1 |
|
|
|
|
|
||
|
Control |
103
|
27.0 |
4.05 |
1 .35 |
2.70 |
||
|
1.0 |
119
|
28.3 |
7.08 |
0.88 |
0.00 |
||
|
2.5 |
96
|
46.3 |
7.25 |
0.00 |
0.00 |
||
|
5.0 |
21
|
61.5 |
7.69 |
0.00 |
0.00 |
||
|
17-35 |
|
|
|
|
|
||
|
Control |
118
|
29.7 |
2.54 |
0.00 |
0.00 |
||
|
1 .0 |
34
|
26.5 |
0.00 |
0.00 |
0.00 |
||
|
2.5 |
42
|
50.0 |
2.40 |
0.00 |
0.00 |
||
|
5.0 |
32
|
37.5 |
6.25 |
0.00 |
0.00 |
||
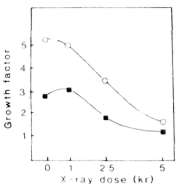
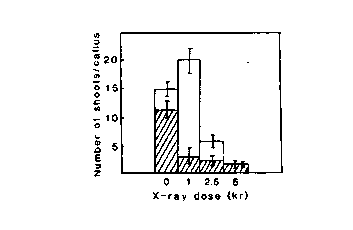
Fig. 1. Influence of the radiation doze on the callus growth
rate of lines 17-35 (square) and 18-1 (circle) in
vitro
Fig. 2. Avarage number of regenerated shoots per X-irradiated
callus of lines 17-35 (empty bars) and 18-1 (filled
bars).