Pisum Genetics
2010-Volume 42
Research Papers
also occur. In the central cylinder, collateral open vascular bundles of
Figure 1. V. formosa stem cross section
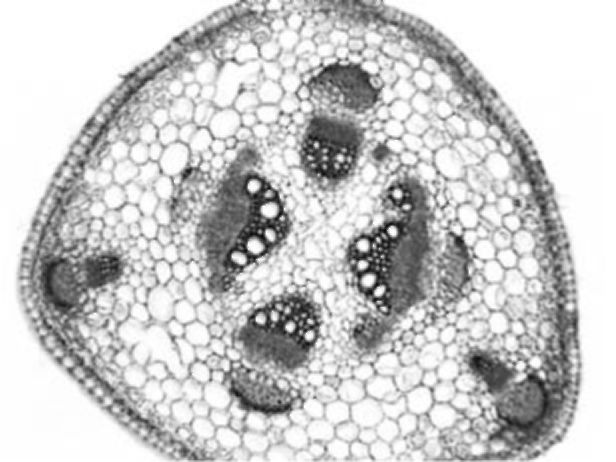
different size are arranged in a circle. Only small groups of sclerenchyma are present near the bundles. Sclerenchyma cells adjacent to the phloem have larger lumen and less lignified walls. Crystals occur in parenchyma sheath cells above the sclerenchyma strands. The central cylinder is composed of parenchyma with no central cavity or sclerenchymatic parenchyma present. Parenchyma cells of the cortex and central cylinder sometimes contain starch grains.
Leaflet. The leaflets of V. formosa have dorsiventral structure (Fig. 2). The
epidermis has a single layer composed of cells with sinuous anticlinal walls and covered with a thick cuticle. It contains large numbers of stomata on both the adaxial and abaxial surface. Stomata are slightly
sunken or at the same level as other epidermal cells. The leaves
Figure 2. V. formosa leaflet cross section
are glabrous on the adaxial side, but rare non-glandular trichomes were observed abaxially along the leaf veins. Leaf photosynthetic tissue is differentiated on palisade and spongy tissue. Palisade tissue is well developed, composed of two to three layers of elongated cells. Under the abaxial epidermis 4-5 layers of spongy tissue cells of irregular shape were observed. Mesophyll cells were closely arranged with small intercellular spaces. Closed collateral vascular bundles were arranged in a single row. Parenchyma sheath cells along the vascular bundles had solitary prismatic crystals. They are larger by the vascular bundle in the main vein which is not very prominent on the abaxial side. Collenchyma tissue is not present or only a few collenchyma cells occur abaxially.
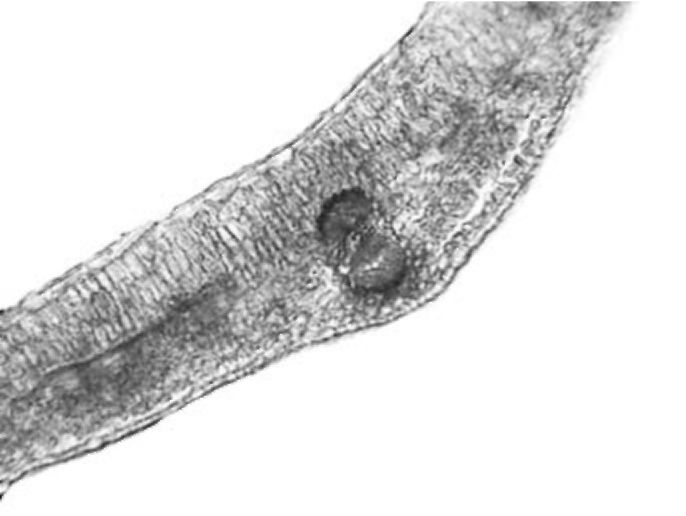
Stipule. Stipules have anatomical structure very similar to the structure of the leaves (Fig. 3). Palisade tissue is not as dominant as the one in the leaves. It is composed of one or two layers of cells. Spongy tissue is present in three to four layers. Larger groups of sclerenchyma tissue are recorded by vascular bundles with solitary crystals in the sheath cells. Stipules are also glabrous.
Figure 3. V. formosa stipule cross section
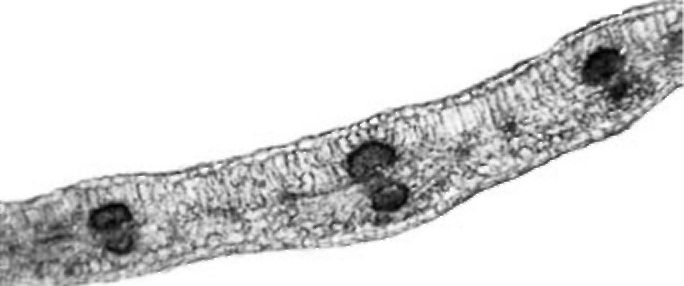
Figure 4. V. formosa petiole cross section
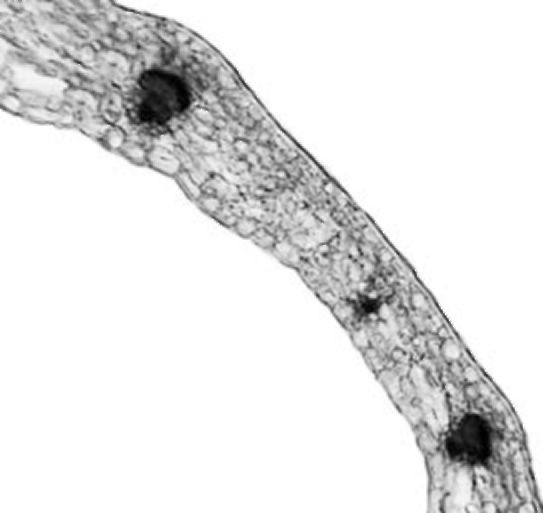
Petiole. The petiole is triangular or heart-shaped in cross section (Fig. 4). The epidermis has a single layer with sparse non-glandular trichomes. Four to five layers of chlorenchyma tissue occur subepidermally. Collenchyma is present in small groups only in the main rib. Five collateral vascular bundles are arranged in the form of an open arc. Sometimes, three vascular bundles in the main ribs are much larger than the other two. Small groups of sclerenchyma tissue occur by the phloem and are somewhat larger by the xylem. Solitary crystals were observed in parenchyma sheath cells above the vascular bundles. The central part of the petiole is filled with parenchyma cells. The central cavity is not present.
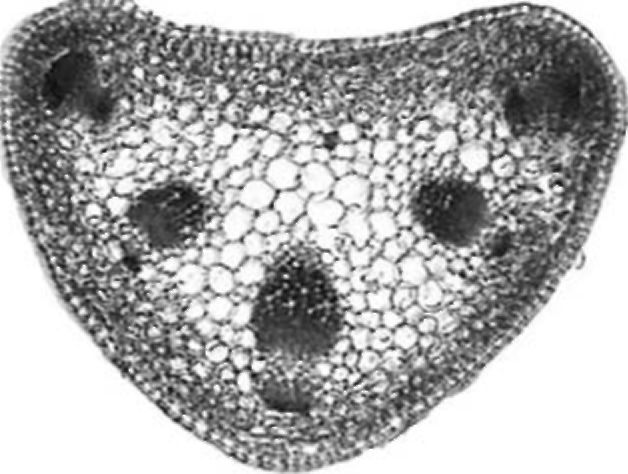
Figure 5. V. formosa rachis tip cross section
Rachis tip. The cross section of a V. formosa rachis tip is elliptical in shape (Fig. 5). On the adaxial side one layer of palisade cells is present. Mesophyll is compact with densely distributed cells.
22