

A revival of the research on beautiful vavilovia (Vavilovia formosa syn. Pisum formosum)
Mikić, A.1, Sm²kal, P.2, 1Institute of Field and Vegetable Crops, Novi Sad, Serbia
Kenicer, G.3, Vishnyakova, M.4 2Agritec Plant Research Ltd., Ŗumperk, Czech Republic
Akopian, J.5, Sarukhanyan, N.6, 3Royal Botanical Garden Edinburgh, Edinburgh, UK
Gabrielyan, I.5, Vanyan, A.6, 4N. I. Vavilov Institute of Plant Industry, St. Petersburg, Russia
Toker, C.7, Ćupina, B.8, 5National Academy of Sciences, Yerevan, Armenia
Ambrose, M.9, Mihailović, V.1 6Green Lane Agricultural Assistance NGO, Yerevan, Armenia
and Ellis, N.9 7Akdeniz University, Faculty of Agriculture, Antalya, Turkey
8University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia
9John Innes Centre, Norwich, UK
Fabeae
The tribe Fabeae (syn. Vicieae) of the family of legumes (Fabaceae Endl) contains more than 300 species. Many of them are economically important, such as common pea (Pisum sativum L.), grass pea (Lathyrus sativus L.), faba bean (Vicia faba L.), common vetch (Vicia sativa L.) and common lentil (Lens culinaris Medik.). These species are used for human consumption and animal feeding, as well as for green manure and other non-food purposes (1).
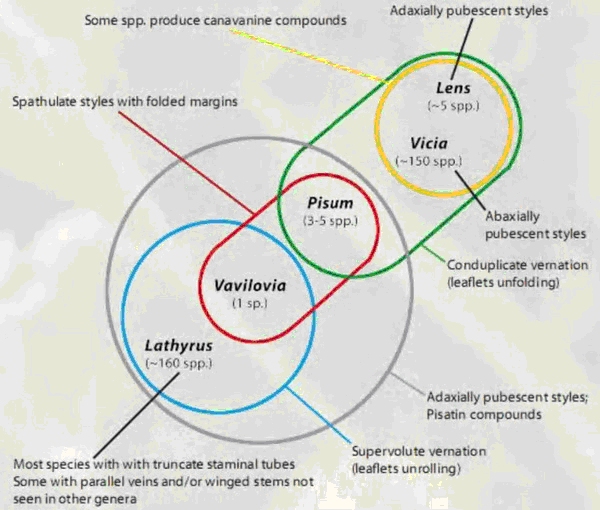
Today, the Fabeae are regarded as comprising five genera. The first four, Lathyrus L. (vetchling) with about 160 species; Lens Mill. (lentil) with 4 species; Pisum L. (pea) with 2 species, and Vicia L. (vetch) with about 140 species, have a well-established taxonomical status. The fifth genera, Vavilovia Fed. (vavilovia), consists of only one species and has been considered being within nearly all four other Fabeae genera. Vavilovia could provide an essential contribution to the present knowledge on the past, present and future of the entire tribe.
Classification
Nearly two hundred years ago, there was a report about a new species of Orobus L. (2), a genus that was subsequently disregarded and its component species moved mostly to Lathyrus. Since its first public appearance in the scientific community this species has been acknowledged for its beauty and was initially named Orobus formosus Stev.
This species was often incorporated within diverse existing genera due to its distinct morphological and ecological characters. Most commonly, it was regarded as a pea species and has been known under the name of Pisum formosum (Stev.) Alef., beautiful or perennial pea (3, 4), P. aucheri Jaub. et Spach., P. formosum Boiss. (5) and P. frigidum Alef. A more recent research of diverse androecium and pistil characters placed it as a pea subgenus (6). It was also regarded as a vetch, named V. aucheri Boiss. and as a vetchling, given the name of L. frigidus Schott & Kotschy.
On the other hand, some classifications treated this species as belonging to a novel monospecific genus. One of them considered it Alophotropis formosa (Stev.) Grossh. (7, 8), but it was another and the older classification (9) that has become most widely accepted today: Vavilovia formosa (Stev.) Fed., beautiful vavilovia, with a name that honours Nikolai Ivanovich Vavilov, who was the first person envisaging and promoting the importance of crop wild relatives.
There have been reports on a certain intraspecific variability, which led to a distinction of few hypothetic subtaxa, such as Orobus formosus var. microphyllus Ser. or Pisum formosum var. pubescens with sparsely pubescent leaflets (10).


Morphology
Beautiful vavilovia is a perennial herbaceous species. In comparison to other Pisum taxa, it has a dwarf habit, with a height between 5 and 15cm. Roots are long, while stems are slender, sprawling or creeping, not winged and with a glabrous surface.
![]() As
in other Fabeae species, the leaf of beautiful vavilovia is compound. Stipules
are small, semi-sagittate, foliaceous and free from the petiole. There is one
pair of
broadly, cuneate-obovate to suborbicular, thick and glabrous
leaflets with non-indentated
margins. The rachis ends with one mucro-like formation, similar to faba bean
(Fig. 1).
As
in other Fabeae species, the leaf of beautiful vavilovia is compound. Stipules
are small, semi-sagittate, foliaceous and free from the petiole. There is one
pair of
broadly, cuneate-obovate to suborbicular, thick and glabrous
leaflets with non-indentated
margins. The rachis ends with one mucro-like formation, similar to faba bean
(Fig. 1).
![]()
The flowers of beautiful vavilovia are usually solitary, axillary and pedunculate. Bracts are small and/or inconspicuous, while bracteoles are not present. Calyx is campanulate, with subequal and narrowly triangular teeth. Corolla is pink or purple (Fig. 2). The standard petal is often oblong, with a short and broad claw, while the wing petal is falcate to oblong and is longer than non-cristate and sometimes white keel. Stamens are monadelphous or diadelphous and anthers are smooth and glabrous. Beautiful vavilovia is considered a cross-pollinating species.

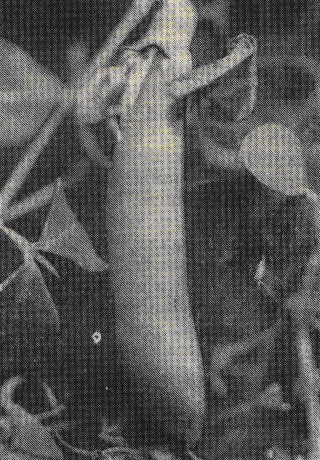
Pods are linear-oblong and dehiscent (Fig. 3), between 20 mm and 35mm long, bear from 3 to 5 seeds per pod (11), globose or oval and smooth, usually with dark blotches on the surface.
Distribution and habitat

The main homeland of beautiful vavilovia is Central and Eastern Caucasus, with a disjunctive area of distribution (12, 13). In the Russian Federation, beautiful vavilovia may be found in isolated habitats in Karachevo-Cherkessia, Cabardino-Balkaria, Northern Ossetia and Dagestan (13). It is also found in other ex-USSR countries, such as Armenia (14), Azerbaijan (15) and Georgia (16, 17), as well as in Iran, Iraq, Lebanon, Syria and Turkey (18).
Beautiful vavilovia is typically found in high-mountain areas, at altitudes from 1500 m up to 3200 m. It prefers shale or rocky ground, such as loose limestone scree. Due to its relic and endemic nature, beautiful vavilovia has been considered an endangered species in most of the countries and is often protected (19, 20).
Genetic resources
For nearly three decades, from 1960 to 1989, several expeditions to collect beautiful vavilovia were carried out in the Caucasus region, mostly in Dagestan, by the N. I. Vavilov Institute of Plant Industry (21). During the last decade there have been several studies on wild flora including beautiful vavilovia in Turkey, such as those on a cedar forest location near Antalya (22) and on a wider region of the Western Taurus Mountains (23). Most recently, there were three expeditions in Armenia during the summer of 2009, in the location of Akna Lich in the mountain ridge of Gehama and the mountains of Ukhtasar. These expeditions were undertaken jointly by the Institute of Botany and Green Lane with promising results in both ecology and conservation of beautiful vavilovia.
There have been many problems related to in situ conservation of beautiful vavilovia, where grazing by both domestic and wild animals, especially goats and sheep, seems to be the most challenging. At the same time, the global climate changes may easily make the suitable environment for it even narrower, possibly leading to its complete extinction in certain countries. In the end, certain physiological peculiarities of this species, such as high susceptibility of flowers to early frosts in late summer and inability of some populations to produce seeds each year, lead to its poor distribution and decreased ability to survive.
![]()

Figure 4. Ex situ conservation of beautiful vavilovia in the Yerevan Botanic Garden
Many attempts have been
made in ex situ conservatio n of beautiful vavilovia,
especially in USSR, and many of them were in vain, mostly due to inadequate
management of soil aeration and water flow (24). Success was achieved in the UK
at the Official Seed Testing Station in Edinburgh and at Southampton University
(25). According to old records, Vavilovia has been periodically in cultivation
at the Flora and Vegetation of Armenia plot of the Yerevan Botanic Garden since
1940 (26). However, true success has been achieved recently in the Yerevan
Botanic Garden (Fig. 4), where several individuals of beautiful vavilovia from
the Akna Lich and Uktasar populations were planted in the rock garden, showing
that under conditions similar to natural habitat this species can be cultivated
and propagated under ex situ conditions (27).
n of beautiful vavilovia,
especially in USSR, and many of them were in vain, mostly due to inadequate
management of soil aeration and water flow (24). Success was achieved in the UK
at the Official Seed Testing Station in Edinburgh and at Southampton University
(25). According to old records, Vavilovia has been periodically in cultivation
at the Flora and Vegetation of Armenia plot of the Yerevan Botanic Garden since
1940 (26). However, true success has been achieved recently in the Yerevan
Botanic Garden (Fig. 4), where several individuals of beautiful vavilovia from
the Akna Lich and Uktasar populations were planted in the rock garden, showing
that under conditions similar to natural habitat this species can be cultivated
and propagated under ex situ conditions (27).
Cytogenetics
Beautiful vavilovia is diploid, with 2n = 14 chromosomes. Early research of its karyotype revealed that it comprised of two submetacentric chromosomes with small-diameter satellites, six submetacentric chromosomes without satellites, two submetacentric chromosomes with satellites of a diameter equal to that of a chromosome itself and four metacentric chromosomes (28).
Hybridization
The same number of chromosomes in vavilovia and its close cultivated relatives, such as common pea, common vetch and grass pea, gave a basis for the attempts of their hybridization. The only known reports of their outcomes are related to common pea and were carried out more than two decades ago within a project by VIR in St. Petersburg and its station in Dagestan (29).

 Hybridization
of ♀
V. formosa x ♂
P. sativum produced several normal seeds. However, only one seed produced a F1
hybrid plant (Fig. 5) that had several basal branches, no lateral branches
typical for beautiful vavilovia, long internodes and leaves where instead of a
terminal mucro-like
organ a third, but smaller leaflet was developed, making its leaves similar to
those of Medicago or Trifolium species. This plant was not able to enter the
generative stage and withered due to chlorosis.
Hybridization
of ♀
V. formosa x ♂
P. sativum produced several normal seeds. However, only one seed produced a F1
hybrid plant (Fig. 5) that had several basal branches, no lateral branches
typical for beautiful vavilovia, long internodes and leaves where instead of a
terminal mucro-like
organ a third, but smaller leaflet was developed, making its leaves similar to
those of Medicago or Trifolium species. This plant was not able to enter the
generative stage and withered due to chlorosis.
Another hybridization of ♀ P. sativum x ♂V. formosa also gave only one F1 hybrid plant. It had significantly greater height in comparison to both parents, numerous basal and lateral branches, flowers and five pods (Fig. 6). All seeds aborted in two pods, while in other three the seeds remained in an immature state.
Unpublished data on the hybridization of beautiful vavilovia with other pea species, such as red-yellow pea (Pisum fulvum Sm.) carried out by A. A. Golubev, reports a chance of success if beautiful vavilovia is used as the male parent (18). The ability to cross beautiful vavilovia and common peas, coupled with a demonstrated susceptibility of beautiful vavilovia to fungal pathogens specific to Pisum, such as Uromyces pisi, Ascochyta pisi and Ascochyta pinodes, was often used to show that its distinction in a separate genera is questionable (30).
Seed protein composition
Generally, the protein composition of beautiful vavilovia has little in common with the one in pea, with globulin, vicine and legumin only sporadically identical, and with much more similarity to that of vetchling species. However, the largest component of the seed protein of beautiful vavilovia belongs to a component absent in either pea or vetchlings (31). This evidence, together with the results of an anatomical analysis showing a partial similarity of Ethiopian pea (P. sativum subsp. abyssinicum Govorov), grass pea and beautiful vavilovia (32), supports the viewpoint that this species belongs to its own genus.
Molecular taxonomy
 So
far, beautiful vavilovia has not been investigated on the molecular level to
conclude anything regarding its definite relationship to the genera of Pisum and
Lathyrus (33). The first attempts were made using herbarium specimens of
beautiful vavilovia from two independent sources, as well as the material
belonging to 30 more species of the tribe Fabeae. The four phylogenetically
informative regions, namely chloroplast maturase K, trn L-F and trn S-G
fragments along with the internal transcribed spacer (ITS) region of nuclear
DNA, were investigated. The maximum parsimony and Bayesian analysis of combined
sequence data confirmed that beautiful vavilovia is a distinct group within a
Lathyrus ¢ Pisum - Vavilovia clade (34). The results support the monophyly of
Pisum and Vavilovia and show that they in turn form a monophyletic pairing (Fig.
7): the Pisum ¢ Vavilovia clade is sister to most of Lathyrus and all are nested
within Vicia (35).
So
far, beautiful vavilovia has not been investigated on the molecular level to
conclude anything regarding its definite relationship to the genera of Pisum and
Lathyrus (33). The first attempts were made using herbarium specimens of
beautiful vavilovia from two independent sources, as well as the material
belonging to 30 more species of the tribe Fabeae. The four phylogenetically
informative regions, namely chloroplast maturase K, trn L-F and trn S-G
fragments along with the internal transcribed spacer (ITS) region of nuclear
DNA, were investigated. The maximum parsimony and Bayesian analysis of combined
sequence data confirmed that beautiful vavilovia is a distinct group within a
Lathyrus ¢ Pisum - Vavilovia clade (34). The results support the monophyly of
Pisum and Vavilovia and show that they in turn form a monophyletic pairing (Fig.
7): the Pisum ¢ Vavilovia clade is sister to most of Lathyrus and all are nested
within Vicia (35).
Significance
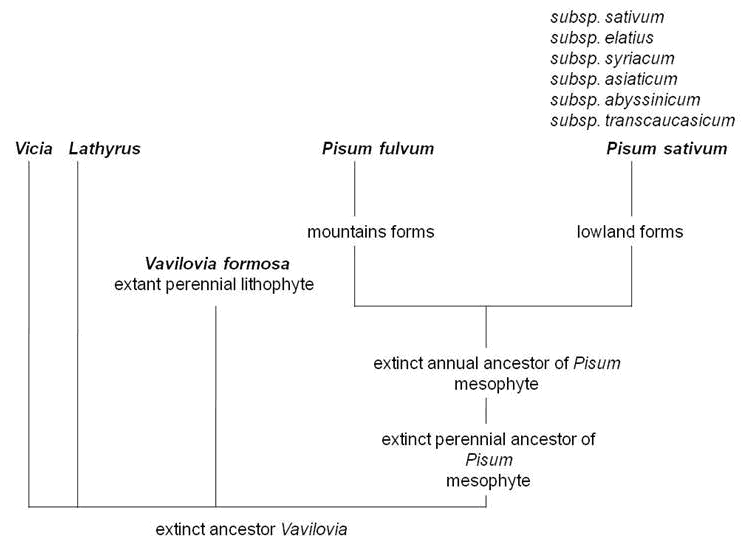
It has been long understood (9), that beautiful vavilovia may reveal much of the evolution of the whole tribe of Fabeae, being possibly the closest of all modern taxa to an extinct common ancestor (Fig. 8). From a breeding point of view and due to a possibility of mutual hybridization with pea, there are ideas that a hypothetical gene for perenniality could be introgressed from beautiful vavilovia into its cultivated relatives. An urgent and concerted effort aimed at the conservation of this species remains a basic and essential precondition for any kind of research, demanding mobilization of researchers belonging to diverse scientific communities and upgraded promotion of the importance of crop wild relatives such as beautiful vavilovia.
 Acknowledgements:
The authors
would like to thank Nigel Maxted for his sincere support in producing this
paper, as well as to dedicate this review in memory of R.H. Makasheva, A.A.
Golubev and all the others who contributed to the research on beautiful
vavilovia.
Acknowledgements:
The authors
would like to thank Nigel Maxted for his sincere support in producing this
paper, as well as to dedicate this review in memory of R.H. Makasheva, A.A.
Golubev and all the others who contributed to the research on beautiful
vavilovia.
Planta formosissima femae formosissimae, Erae Leguminosarum nomini.
1. Mikić, A., Ćupina, B., Katić, S. and Karagić, ą. 2006. A Periodical of Scientific Research on Field and Vegetable Crops 42:I:91-103.
2. Steven, C. 1812. Mķmoires de la Sociķtķ Impķriale des Naturalistes de Moscou 4:50.
3. Alefeld, F. 1861. Bonplandia 9:327.
4. Alefeld, F. 1866. Landwirtschaftliche Flora, Verlag Wiegand und Hempel, Berlin, Germany, pp. 360.
5. Boisser, E. 1872. Flora Orientalis 2:624.
6. Gunn, C.R. and Kluve, J. 1976. Taxon 25:5/6:563-575.
7. Grossheim, A.A. 1949. OpredelitelÆ rastenii Kavkasa. Sovetskaya Nauka, Moscow, Russia, 162.
8. Lamprecht, H. 1972. Monographie der gattung Pisum, Steiermarkische Landesdruckerei, Graz, Austria, pp. 665.
9. Fedorov, A.A. 1939. Trudy Biol. Inst. Arm. Fil. Akad. Nauk S.S.S.R. 1:52.
10. Townsend, C. C. 1968. Kew Bulletin 21:455.
11. Davies, P.H. 1970. In: Flora of Turkey and East Aegean Islands 3, Edinburgh, UK, pp. 44-45.
12. Galushko, A.I. 1980. Flora of Northern Caucasus - A field guide 2, Rostov-on-Don, Russia, pp. 352.
13. Grossheim, A.A. 1952. In: Flora of the Caucasus 5, AN USSR, Moscow ¢ St. Petersburg, Russia, pp. 414-416.
14. Gabrielyan, E.Ts. 1962. In: Flora of Armenia 4, Yerevan, Armenia, pp. 322.
15. Carjagin, I.P. 1954. Flora of Azerbaijan 5, AN, Baku, Azerbaijan, pp. 580.
16. Arabuli, G. 1981. Notulae systematicae ac geographicae Instituti Botanici Tbilissiensis 37:16-18.
17. Kolakovskiy, A.L. 1958. Flora of Abhasiya 3, Tbilisi, Georgia.
18. Maxted, N. and Ambrose, M. 2000. In: Plant Genetic Resources of Legumes in the Mediterranean, Kluwer, Dordrecht, The Netherlands, pp. 181-190.
19. Gabrielyan, E.Ts. 1990. In: Red Data Book of Armenia, Yerevan, Armenia, pp. 123.
20. Popov, K.P. 1988. In: Red Data Book of the Russian Federation, Pedagogika, Moscow, Russia, pp. 235-236.
21. Vishnyakova, M., Yan'kov, I., Mikić, A. and Ćupina, B. 2007. Book of Abstracts of the 6th European Conference on Grain Legumes, Lisbon, Portugal, 12-16 November 2007, pp. 122-123.
22. Deniz, İ.G. and S³mb³l, H. 2004. Turkish Journal of Botany 28:529-555.
23. Eren, ų., G÷kńeoğlu, M. and Parolly, G. 2004. Willdenowia 34:463-503.
24. Makasheva, R.Kh. 1973. Gorokh, Kolos, St. Petersburg, Russia, pp. 312.
25. Cooper, S.R. and Cadger, C.A. 1990. Pisum Newsletter 22:5.
26. Akhverdov A.A. and Mirzoeva, N.V.. 1949. BulletenÆ Botanicheskogo Sada Akademii Nauk Armianskoy SSR 8:37-45.
27. Akopian, J. A. and Gabrielyan, I.G. 2008. Crop Wild Relative 6:26-27.
28. Abramova, L.I. 1971. Trudy po prikladnoi botanike, Genetike i Selektsiyi 45:3: 240-243.
29. Golubev, A.A. 1990. Trudy po prikladnoi botanike, Genetike i Selektsiyi 135: 67-75.
30. Yan'kov, I. and Golubev, A.A. 1999. CD of the XVI International Botanical Congress, St. Louis, USA, 1-7 August 1999, p. 5655.
31. Makasheva, R.Kh. 1979. Flora of Cultivated Plants IV, Grain Legumes 1, Pea, Kolos, St. Petersburg, Russia, pp. 321.
32. Makasheva, R.Kh., Drozd, A.M., Adamova, O.P. and Golubev, A.A. 1973. Trudy po prikladnoi botanike, Genetike i Selektsiyi 51:1:44-56.
33. Kenicer, G.J., Sm²kal, P., Visnhakova, M. and Mikić, A. 2009. Grain Legumes 51:8.
34. Kenicer, G.J., Sm²kal, P.and Mikić, A. 2008. Abstract Book of IV International Conference on Legume Genomics and Genetics, Puerto Vallarta, Mexico, 7-12 December 2008, p. 83.
35. Sm²kal, P., Kenicer, G.J. and Mikić, A. 2009. Book of Abstracts of the IV Congress of the Serbian Genetic Society, Tara, Serbia, 1-5 June 2009, p. 166.
36. Kenicer, G.J., Kajita, T., Pennington, R.T. and Murata, J. 2005. Am. J. Bot. 92:1199-1209.
37. Makasheva, R.Kh. 1975. In: Genetika i Selektsiya Gorokha, Nauka, Novosibirsk, Russia, pp. 5-15.