Comparison of genetic maps for
two
related pea populations (Pisum sativum L.)
Gawłowska,
M., Święcicki, W. and Wolko, B.
Inst. of Plant Genet.
Polish Acad. of Sci.,
Poznań
,
Poland
Introduction
Genetic
maps can serve many plant breeding purposes, such as tagging single genes or
permitting the localization of quantitative trait loci (QTL).
Such associations between markers and traits are useful for following the
trait in the segregating progeny. However,
it is not possible to create a map for each breeding population. The most
valuable map is thus one with markers transferable to populations other than the
mapping population. Here we compare two linkage maps developed for two related
populations with one common parent in order to compare the reliability of marker
order and distances between markers.
In
addition, different computer packages have been used for linkage analysis, e.g. Mapmaker/Exp.
and JoinMap. Both use pairwise
recombination rate to find marker order and to estimate the distances between
marker loci. However, these two programs give somewhat different results.
The linkage map for Wt 11238
(tester line)
x Wt
3557 (cv. Paloma) population has been constructed using the maximum likelihood
method (Mapmaker/Exp. v. 3.0) and the least squares (regression method) (JoinMap
v.3.0 software). Of the markers analyzed 72% were AFLPs, 13% RAPDs, 4%
morphological characters, 4% isozymes, 4% ISSRs, 2% CAPSs and 1% STSs. The
results obtained from these two approaches were compared. Two versions of the
map spanned 2172 cM and 841 cM respectively. The average distance between
adjacent markers was 22 cM and 10 cM. The comparison was also made with the map
published earlier for the Wt 10245 x Wt
11238 population (one parent in common).
Materials
and methods
Plant material
Two populations from the cross Wt11238 x Wt3557 were
investigated: an F2 population of 116 plants and an F4
population of 108 plants. The Wt3557 parental line is the cultivar Paloma and
the Wt11238 (=WL1238 or NGB1238) is a tester line. Parental
lines were obtained from the Pisum
Gene Bank at Wiatrowo. The second cross involved the parental lines Wt10245
x Wt11238.
The map for this population has been described by Irzykowska et al. (2) and included 204 markers and 9
linkage groups. The length of the map was determined to be 2416 cM with an
average distance between adjacent markers of 12 cM.
Map construction and comparison of linkage groups
For
the linkage analysis in the Wt11238 x Wt3557
populations, DNA
markers (AFLP, RAPD, ISSR, CAPS, STS) as well as morphological markers (a,
b, D,
tl, gp, cp,
te, i,
r, Fs)
and allozyme loci (Acp1, Lap1, Lap2, Aat-m,
Aat-p, Idh, Est2,
Est1, Gal2, Gal3)
were used (Irzykowska et al. 2001). Goodness-of-fit to the codominant
1:2:1 or dominant 3:1 ratio was tested by the c2
analysis using the computer program JoinMap (11). The genetic map construction
was performed by the MAPMAKER/EXP v. 3.0 software as previously described (3). The Haldane function was used.
For the population Wt10245 x Wt11238, the analysis of markers was performed as
described in (2).
The reconstruction of linkage groups was made using the Join Map v. 3.0
computer program. Segregated markers were grouped and distances among them were
calculated. For all linkage groups a minimum LOD score was 1.00, LOD for
grouping = 3.0 or 4.0, recombination rate < 0.4, ripple value = 1, jump
threshold = 5.0. The jump threshold delays the inclusion of a marker in the
first two rounds to the next round, if this marker raises the chi2
value of the lack of fit by more than the jump threshold. The pairwise
recombination frequencies were estimated and the corresponding LOD values were
calculated. If several estimates of the recombination frequency between a pair
of markers were available they were replaced by a single value after appropriate
weighting (9). The Haldane mapping function was chosen. The ōfixed orderö
option was taken to order the reference markers.
Maps from both populations can be compared if common and reference
markers exist. The term ōreference markersö stands for the markers, placed
on the map, published by the Pisum
Mapping Committee (14) and the map with expressed sequence tags (EST) (1).
Segregation of these markers was observed in analysed mapping populations
(Wt10245 x Wt11238 and Wt11238 x Wt3557).
The term ōcommon markersö stands for markers revealing polymorphism in
both populations. The number of common markers for the linkage groups are given
in Table 1.
Table
1. Number of reference and common markers for investigated pea populations
|
|
Wt11238 x Wt3557
|
|
Wt10245
x Wt11238
|
|
Linkage
group
|
reference
markers
|
common
markers
|
reference
markers
|
|
I
|
4
|
4
(2)
|
3
|
|
II
|
4
|
4
(2)
|
5
(4 in JoinMap)
|
|
III
A
|
2
|
1
(1)
|
4
|
|
III
B
|
2
|
2
(2)
|
|
IV
|
1
|
1
(1)
|
3
|
|
V
A
|
2
|
3
(2)
|
10
(9 in JoinMap)
|
|
V
B
|
5
|
3
(3)
|
|
VI
A
|
1
|
2
(1)
|
1
|
|
VI
B
|
-
|
1
|
|
VII
A
|
1
|
-
|
3
|
|
VII
B
|
-
|
2
|
In parentheses: reference markers which were common.
Results
and Discussion
The genetic linkage map for Wt 11238 x Wt 3557
population comprised 86 markers (100 in Mapmaker/Exp.) with average distance 10
cM between markers (22 cM in Mapmaker/Exp.) and total length 841 cM (2172 cM in
Mapmaker/Exp.). The number of polymorphic markers, population description and
comparison with earlier described Wt 10245 x Wt 11238
population is presented in Table 2.
Table 2. Description of pea populations used for
mapping
|
|
Wt 11238 x Wt
3557
|
Wt 10245 x Wt 11238
|
|
 Characteristic Characteristic
|
Mapmaker
|
 JoinMap JoinMap
|
Mapmaker
|
|
Population
size
|
116 F2
108 F4
|
114 F2
104 F4
|
|
Total
number of polymorphic markers
|
264
|
240
|
|
Number
of linked markers
|
100
|
86
|
204
|
|
AFLP
|
51 (9)
|
41 (11)
|
140
|
|
RAPD
|
23
|
20
|
24
|
|
ISSR
|
4
|
3 (1)
|
10
|
|
CAPS
|
4
|
4
|
5
|
|
STS
|
3
|
3
|
1
|
|
isozymes
|
5
|
5
|
11
|
|
morphological
|
10
|
10
|
13
|
In parentheses: markers with distorted segregation.
The population size and total marker number was similar to those from
Wt10245 x Wt11238 population. However, the large number of unlinked markers was
characteristic for Wt11238 x Wt3557 population (version in Mapmaker/Exp. ¢ 56%
unlinked markers, version in JoinMap ¢ 67% unlinked markers). The described
level is higher than reported earlier (2 ¢ 15% in pea, 7 ¢ 19% in other
legumes) (2, 5, 6, 8, 10). One of the possible reasons is the construction
method for the mapping population. DNA was isolated from five randomly chosen
plants of the F4 generation, which were an offspring of each ancestor
F2 plant. DNA was combined in bulk. The observation was written down
as F2 plant data, but after 3 meiotic cycles, not after 1. The
probability of recombination has been increased. The inspection in Wt10245 x Wt11238 population was made in the F3 generation (after 2
recombination cycles), so the level of unlinked markers was lower. Random
selection of 5 plants from the F3 generation changed the assessment
of the recombination frequency. Another choice in the next generation may
increase this error. The high level of unlinked markers could be caused by
considerable involvement of dominant markers among total analyzed markers. AFLP,
RAPD and ISSR markers constituted of 94% markers.
Two individual maps for Wt11238 x Wt3557 population were generated using two programsŚ
Mapmaker/Exp. and JoinMap. The identity of markers in both versions of the map
was checked and presented in Table 3.
Table
3. The percentage of identical markers in both versions of the map for Wt 11238
x Wt 3557
|
Linkage group
|
marker
number
|
Mapmaker
|
JoinMap
|
marker
number
|
|
I
|
15
|
100 % identical markers like in JoinMap
|
94 % identical markers like in Mapmaker
|
16
|
|
II A
|
7
|
100 % identical markers
|
12
|
|
II
B
|
5
|
100 % identical markers
|
|
III A
|
12
|
75 % identical markers
|
100 % identical markers
|
9
|
|
III
B
|
8
|
100 % identical markers
|
88 % identical markers
|
9
|
|
IV
|
6
|
83 % identical
markers
|
100 % identical
markers
|
5
|
|
V
A
|
5
|
100 % identical markers
|
5
|
|
V
B
|
27
|
59 % identical
markers
|
100 % identical
markers
|
16
|
|
VI
|
7
|
100 % identical
markers
|
87 % identical markers
|
8
|
|
VII
A
|
4
|
100 % identical
markers
|
50 % identical
markers
|
2
|
|
VII B
|
4
|
100 % identical
markers
|
4
|
In most cases the same
markers were included in the map in both programs. The reference marker order
was consistent with this presented by the reference maps (1, 14). If any
discrepancies were observed, one of the solutions was applied depending on the
software used. The marker order can be changed and corrected by a user in the
Mapmaker/Exp. This option is not available in JoinMap. The user is able to fix
the order of markers (option ōfixed ordersö). However, if the order is
significantly inconsistent, the marker introduction into specific group
doesnÆt proceed (13). In some cases the ōfixed ordersö option had to be
used regarding reference markers to keep agreement of marker order with the map
published by the Pisum Mapping Committee and the linkage map with EST (1, 14). This
option was applied in the analysis of LG I, II, III in the population Wt 11238 x Wt 3557 and LG
I, II, III, V in the Wt 10245 x Wt 11238 population. The application of the option
ōfixed ordersö can cause the reference markers to be deleted from the
analysis. Such a deletion occurred for the reference marker ōsö in
the second linkage group of the map of population Wt 10245 x Wt 11238. The
final map for Wt11238 x Wt3557 obtained in JoinMap is presented in Fig. 1. The reference markers
from the map published by the Pisum
Mapping Committee and from the linkage map with EST were marked in bold, and the
markers common with Wt10245 x Wt11238 were marked in a box.
Different maps can be compared when common polymorphic markers exist.
AFLP fragments obtained using the same primer combinations and displaying
the same molecular weight, are generally thought to be homologous (12). Our
comparison of the AFLP profiles of two mapping populations revealed 21 DNA
fragments that appeared to be segregating in both populations. After
construction of the Wt11238 x Wt3557 reference markers from the map
published by the Pisum Mapping
Committee and from the linkage map with EST were marked in bold, and the markers
common with Wt10245 x Wt11238 were marked in a box.

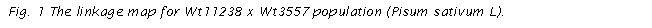
Different maps can be compared when common polymorphic markers exist.
AFLP fragments obtained using the same primer combinations and displaying
the same molecular weight, are generally thought to be homologous (12). Our
comparison of the AFLP profiles of two mapping populations revealed 21 DNA
fragments that appeared to be segregating in both populations. After
construction of the Wt11238 x Wt3557 map and a
comparison with the Wt10245 x Wt11238 map, eight of these were placed in the
corresponding linkage groups in both populations. The remaining common AFLP
markers did not show any linkage in Wt11238 x Wt3557
population.
The heterogeneity test was performed to allow
comparison of the recombination rates between common markers. The test threshold
value was χ2=3.84 for error probability P=0.05
and 6.63 for error probability P=0.01 and for 1 degree of freedom. All values
were not skewed significantly except the recombination rate between marker pairs
in LG II and LGV. Four common markers in LG II were arranged in six possible
pairs and two of these tests were significant (a
and afp15h - χ2=11.89,
and a and afp5d, χ2=4.87). Three common markers in LG VB were composed in possible pairs,
and one test was significant (te and
gp, χ2=10.74), reflecting a difference in marker order in the
different populations (te ¢ gp ¢ cp
in Wt11238 x Wt3557 population, te
¢ cp ¢ gp in Wt10245 x Wt11238 population). The significant value for the
heterogeneity test probably was produced by an inaccuracy in one of the
individual maps. Such inconsistencies make the map comparison and map possible
integration more difficult.
Table
4 summarizes the results of mapping for populations Wt11238
x Wt3557
and Wt10245 x Wt11238
with Mapmaker/EXP 3.0 and JoinMap 3.0.
The
lengths of individual linkage groups in Mapmaker/Exp. varied from nearly equal
to 5.5 times longer compared with the analogous groups created by JoinMap. The
total map length in Mapmaker/Exp. was 2.5 times longer than the total map length
in JoinMap for both populations. The same mapping function was used. This
dissimilarity results not only from the different number of markers in the
related groups but also from the distinct calculation approach in both programs.
The Mapmaker calculates the map length as the sum of adjacent distances. This
method assumes an absence of interference. JoinMap uses all pairwise estimates
for calculating the total map length. The interference is taken into account (4,
7).
Table
4. The comparison of a linkage group length and marker numbers in both
populations created by Mapmaker/EXP 3.0 and JoinMap 3.0
|
linkage
group
|
Population
|
|
 Wt 11238 x Wt 3557 Wt 11238 x Wt 3557
|
Wt
10245 x Wt 11238
|
|
 Mapmaker
/ Exp. Mapmaker
/ Exp.
|
 JoinMap JoinMap
|
 Mapmaker
/ Exp. Mapmaker
/ Exp.
|
JoinMap
|
|
length
(cM)
|
marker
number
|
length
(cM)
|
marker
number
|
length
(cM)
|
marker
number
|
length
(cM)
|
marker
number
|
|
I
|
285
|
15
|
126
|
16
|
388
|
37
|
99
|
36
|
|
II
A
|
120
|
7
|
97
|
12
|
341
|
30
|
220
|
31
|
|
II
B
|
92
|
5
|
|
III
A
|
303
|
12
|
82
|
9
|
504
|
37
|
140
|
37
|
|
III
B
|
154
|
8
|
107
|
9
|
|
IV
|
122
|
6
|
75
|
5
|
256
|
20
|
83
|
21
|
|
V
A
|
74
|
5
|
57
|
5
|
351
|
34
|
199
|
27
|
|
V
B
|
765
|
27
|
139
|
16
|
|
VI
A
|
80
|
7
|
83
|
8
|
185
|
15
|
63
|
7
|
|
VI
B
|
83
|
3
|
33
|
3
|
|
VII
A
|
83
|
4
|
45
|
4
|
276
|
22
|
¢
|
¢
|
|
VII
B
|
94
|
4
|
30
|
2
|
36
|
5
|
|
75
|
16
|
|
total:
|
2172
|
100
|
841
|
86
|
2384
|
198
|
948
|
183
|
The
stable map should present the final version similar and independent of the
calculation approaches and the parameter settings (13). The differences in group
length and marker number were observed for two versions of the map for
populations Wt11238
x Wt3557 and Wt10245 x Wt11238. The reference marker order was consisted with
this published (1, 14).
1. Gilpin, B.J.,
McCallum, J.A., Frew, T.J. and Timmerman-Vaughan, G.M. 1997.
Theor. Appl. Genet. 95: 1289-1299.
2. Irzykowska, L., Wolko,
B. and Święcicki, W.K. 200.
1 Pisum Genetics 33: 13-19.
3. Lander, E.,
Green, P., Abrahamson, J., Barlow, A., Daley, M., Lincoln, S. and Newburg, L. 1987.
Genomics 1: 174-181.
4.
Liebhard, R., Koller, B., Giantfranceschi, L. and Gessler, C. 2003.
Theor. Appl.
Genet. 106: 1497-1508.
5.
Menendez, C.M., Hall, A.E. and Gepts, P. 1997.
Theor. Appl. Genet. 95: 1210-1217.
6.
Pilet-Nayel, M., Muehlbauer, F., McGee, R., Kraft, J., Baranger, A. and Coyne, C. 2002.
Theor. Appl Genet. 106: 28-39.
7.
Qi, X., Stam, P. and Lindhout, P.
1996. Genome
39: 379-394.
8. Roman, B.,
Satovic, Z., Rubiales, D., Torres, A. and Cubero J. 2001.
In: Towards the sustainable production of healthy food, feed and novel
products. Proc. of the 4th European Conference on Grain Legumes. Krak¾w, pp
14-15.
9. Stam, P.
1993. Plant
J. 3: 739-744.
10.
Timmerman-Vaughan, G.M., McCallum, J.A., Frew, T.J., Weeden, N.F. and Russell, A.C. 1996.
Theor. Appl. Genet. 93: 431-439.
11.
Van Ooijen, J. and Voorrips, R. 2001. Plant
Research International B.V. Wageningen,
The
Netherlands
.
12. Van der Voort, J., van Zandvoort,
P., van Eck, H., Folkertsma, R., Hutten, R., Draaistra, J., Gommers, F.,
Jacobsen, E., Helder, J. and Bakker, J. 1997.
Mol. Gen. Genet. 255: 438-447.
13.
Weber, W., Borchardt, D. and Koch, G. 1999.
Plant Breeding, 118: 193-204.
14.
Weeden, N.F., Ellis, T.H., Timmerman- Vaughan, G.M., Święcicki, W., Rozov, S.M. and Berdnikov, V.A. 1998
Pisum Genetics 30: 1-4.
![]() Characteristic
Characteristic
![]() JoinMap
JoinMap
