 Results
Results
Extending MarxÆs isogenic lines in search of Uni function
DeMason, D.A. Dept. of Bot. and Plant Sci.
Introduction
The backcross method of breeding is used widely to improve self-pollinating crop plants and to produce isogenic lines (1). Isogenic lines are inbred lines that are generally superior in quality but deficient in one or only a few specific traits. To produce isogenic lines, plants with an allele or alleles to be substituted are successively backcrossed to the superior parent (recurrent parent). This results in a stock with exactly, or nearly exactly the genotype of the recurrent parent except for the trait or traits of interest. Six rounds of backcrosses with the recurrent parent is the generally accepted standard in backcross breeding programs of self-pollinating species (1). Marx (13) produced a set of eight isogenic lines for three leaf form mutations (afila - af, tendrilless - tl, stipuleless - st, afila tendrilless, afila stipuleless, tendrilless stipuleless, afila tendrilless stipuleless, and Afila Tendrilless Stipuleless - WT). These lines are available from the Marx collection at the USDA Western Regional Plant Introduction Station (http://www.ars-grin.gov/ ars/PacWest/Pullman/GenStock/pea/MyHome.html). I report here that I have extended this set of isogenic lines by adding four lines incorporating the tendrilled-acacia (uni-tac) mutation to create uni-tac, afila uni-tac, tendrilless uni-tac, and afila tendrilless uni-tac. The leaf phenotypes of these lines are also described and compared.
Materials and Methods
Plant materials
The recurrent parents were W6 22593 (WT),
W6 22594 (tl), W6 22597 (af),
and W6 22598 (af tl). The donor parent
was W6 15272 (uni-tac).
This latter line was obtained from the Marx collection and the others
were obtained from
Backcross breeding procedures
The donor parent was backcrossed to the recurrent parents. Twenty pollinations were done for each combination and the resulting F1 seeds were pooled. F2 plants with the uni-tac phenotype were selected from each backcross before repeating the pollinations. Six backcrosses were made in total for each combination. The uni-tac lines were used as the female parent for five of these backcrosses and as the male parent for one backcross for each combination to remove any maternal effects of the original donor parent. BC6F3 seeds from the sixth backcross for each new genotype were propagated.
Leaf form comparisons
Sixteen plants of each of the eight genotypes were grown in a standard greenhouse in the spring (long days). After flowering, the leaf from the last non-flowering node of each plant was analyzed and some of these were removed and photographed. Analysis consisted of counting, and identifying the number and form of each pinna in each pair on the leaf. Pinna position was numbered in order from lamina base to tip and only completely free pinnae were counted for the uni-tac genotypes as described in DeMason and Schmidt (5). Photographs of leaves were taken on a standard copy stand with a Canon Powershot G3 camera. Plates were assembled with Adobe Photoshop 6.
Means and standard errors of pinna pair numbers present on leaves of each genotype were calculated in Microsoft Excel XP and graphed with SigmaPlot 2000. T-tests were done between all relevant pairs of genotypes using Microsoft Excel XP.
 Results
Results
Leaf phenotypes
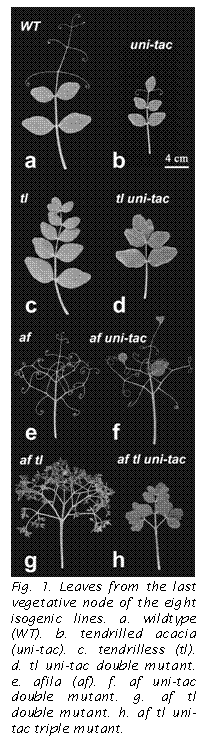
WT - All leaves on the last vegetative node were identical for all sixteen plants. Each had five lateral pinna pairs and a terminal tendril. The lateral pinna pairs consisted of two pairs of leaflets and three pairs of simple tendrils (Fig. 1a).
uni-tac - All leaves on this genotype had terminal leaflets. Thirteen leaves had two pairs of leaflets and one pair of tendrils (Fig. 1b). Two leaves had 2.5 lateral pairs and these were two pairs of leaflets and one lateral, unpaired tendril. One leaf had two pairs of lateral leaflets.
tl - All pinnae, both lateral and terminal, were leaflets (Fig. 1c). Eleven leaves had five lateral leaflet pairs, three had five pairs and one unpaired leaflet, and two had six pairs.
tl uni-tac - All pinnae on this genotype were also leaflets. The number of pinna pairs on the last vegetative leaf ranged from two to three. Many of the leaves that had only 2 pairs of lateral leaflets had bilobed terminal leaflets (Fig. 1d).
af ¢ Leaf form on this genotype has been carefully described previously (4). Briefly, leaves on this genotype possess only tendrils, but there are two types: compound and simple. Adult leaves typically possess 2-3 pairs of compound tendrils in the proximal region and 3 pairs of lateral, simple tendrils in the distal region of the blade (Fig. 1e).
af uni-tac - All leaves had terminal leaflets and most of these were abnormal in size and shape. They differed from typical leaflets on WT and tl plants in that they had elongated, tapering bases that lacked pulvini and were somewhat thigmotropic and had rounded or truncated leaflet tips (Fig. 1f, terminal pinna). The number of lateral pinna pairs on this genotype ranged from three to four. Fifteen out of sixteen leaves had compound pinnae at position 1. One leaf had a normal leaflet opposite a compound pinna at this position. Compound pinnae terminated with either a simple tendril or an abnormal leaflet. Seven leaves had compound pinna pairs at position 2; four had a normal leaflet opposite a compound pinna (Fig. 1f); and six had a leaflet pair. All of these leaflets were normal in size and shape. Pairs of simple tendrils were present at all distal, lateral pinna positions.
af tl ¢ Leaf form on this genotype has been described previously (17). All leaves on these plants had 6 lateral pinna pairs. The terminal pinna was a leaflet on all leaves. The first five pinna pairs were all compound. On eleven leaves the last lateral pinna position consisted of a pair of leaflets (Fig. 1g). On three it was a leaflet opposite a compound pinna. One leaf had a pair of compound pinnae at this distal-most lateral position.
af tl uni-tac - All leaves on this genotype had terminal leaflets. All leaves except one (i.e. 15 out of 16) had a pair of compound pinnae at position 1. The odd leaf had a leaflet opposite a compound pinna. Fourteen leaves had a pair of leaflets at position 2 (Fig. 1h). Two leaves had a leaflet opposite a compound pinna at position 2. All other positions had leaflets on all leaves. The leaflets on this genotype were much larger than those on af tl.
Number of lateral pinna pairs
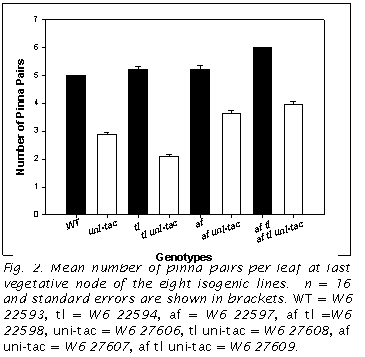
The mean number of lateral pinna pairs differed between these various genotypes (Fig. 2). WT had 5, whereas af tl had 6. tl and af each had means of 5.2. In t-test comparisons with WT, the only pair not found to be different was WT vs af (P=0.065). All the uni-tac lines had lower means compared to their closest genotypes. These were all significantly different at the 0.01 level. This reduction of the mean number of pinna pairs was 43% for uni-tac compared to WT. Reduction was less for the af genotypes: 30% and 34% for af uni-tac vs. af and af tl uni-tac vs. af tl, respectively. Finally, there was a 60% reduction in tl uni-tac vs. tl.
Discussion
The ōtacö allele and its phenotype were first identified and described by Sharma (14). This mutant was produced by diethyl sulfate treatment. According to Sharma, the leaf phenotype possesses lateral and terminal leaflets and the presence or absence of a pair of subterminal, lateral, simple tendrils. Marx (11) determined that the mutation known as unifoliata (uni) and ōtacö (hence uni-tac) are allelic and both he and Sharma (15) crossed uni-tac with other foliar mutants to observe gene interactions. Their descriptions were rather cursory and the lines were not isogenic. However, Marx (12) concluded that uni-tac affects the distal lateral and terminal pinna positions and tends to reduce ōramificationsö of the pea leaf and Sharma (15) concluded that the uni-tac allele converts the terminal pinna into a full-sized leaflet and suppresses differentiation of lateral tendrils. Also, using non-isogenic lines, DeMason and Schmidt (5) did a careful morphological analysis of leaf form and used the SEM to observe the development of uni, and uni-tac leaves compared to WT. They concluded that the lateral pinnae on these genotypes correspond to the proximal-most pinna pairs on WT leaves; whereas the terminal leaflet corresponds to the missing distal tendril pairs and the terminal tendril on WT leaves. uni and uni-tac leaves have a truncated period of leaf development due to precocious differentiation of the terminal leaflet, which explains the reduced number of pinna pairs (5). Therefore, the role of the Uni gene is to promote continued tip growth of pea leaf primordia to allow for a longer period of pinna initiation and the production of larger and more complex compound leaves.
Hofer et al. (9) determined that the Uni gene in pea is the ortholog of the Floricaula(Flo)/LEAFY(LFY) gene and obtained the sequence of two uni alleles. Both of these have defects in the coding sequence. DeMason and Schmidt (5) sequenced the uni-tac allele from the Marx collection and found a normal coding sequence but extremely low transcript levels. Using semi-quantitative RT-PCR, DeMason and Chawla (3, 4) showed that shoot tips of the various leaf form mutants have differential expression of Uni. Uni mRNA levels are much more abundant in af (4.7X) and af tl (10X) but less abundant in uni-tac (0.15X) and uni (0.6X) than in WT (1X) (3). Further, af uni-tac and af tl uni-tac have reduced Uni mRNA levels compared to af and af tl (4). Therefore, for uni-tac, reduced Uni mRNA levels correlates with leaf form simplifications.
Uni is the most important gene for controlling the compound nature of pea leaves. Busch and Gleissberg (13) have proposed that two redundant pathways for leaf dissection (KNOX1 and FLO/LFY/UNI) characterize the ancestral condition in eudicots with compound leaves. KNOX over-expression increases the branching in tomato leaves. Both mechanisms have been retained in some lineages, whereas one or the other has been lost in others. Pea provides the best model system for studying the role of FLO/LFY/UNI in the development of compound leaves.
The goal of this project was to create isogenic lines of uni-tac to extend the set already constructed by Marx (13). Using these genetic resources, researchers can better determine gene interactions underlying the resulting leaf phenotypes and study the underlying molecular regulation. I also provide a careful analytical comparison of the adult leaves in the eight isogenic lines.
Leaf forms of the uni-tac and tl uni-tac genotypes in this new set of isolines are as expected. There are fewer lateral pinna pairs present and the ones present are similar in form and size to the lower-most ones on WT and on tl. And the terminal pinna is a leaflet. However, leaf forms of the af uni-tac and af tl uni-tac have some novel characteristics. The af uni-tac genotype has two types of leaflets, neither of which occurs on af. Leaflets that form directly on the rachis have normal sizes and shapes, but those at the leaf tip or the tips of compound pinnae have abnormal characteristics. The bases of these leaves are similar to tendrils in that they are elongate, lack pulvini and are slightly thigmotropic; whereas the tips are flattened and expanded like leaflets. Terminal leaflets fused to lateral tendrils on uni-tac plants have a similar morphology (pers. observ.). I interpret these abnormal leaves on the af uni-tac plants to be an intermediate between tendrils and leaflets: the bases are more tendril-like and the tips are more leaflet-like. These intermediates are quite different in form from the intermediates present on Tl/tl heterozygotes which have flattened tendrils or narrow leaflets as described previously (10, 17). The position and number of leaflets (both types) present on adult leaves of the af uni-tac genotype are quite variable from leaf to leaf. The number of leaflets present on the leaves varies at different stages of plant ontogeny as well. The leaves at the lower, juvenile nodes have relatively more leaflets than do adult leaves, or those subtending axillary inflorescences. As the plants get older the leaves they produce are more like those on af plants. This feature of uni-tac plant ontogeny was observed previously (7).
The af tl uni-tac genotype also has an unexpected feature. Although essentially all lateral pinnae on af tl leaves are compound, only the lowermost pinna pair on af tl uni-tac is consistently compound. Therefore, the pinna type at position 2 of this genotype is not similar to that of af tl. The two af genotypes used in this study have higher levels of Uni mRNA in their shoot tips because the developing compound pinnae express the gene, whereas no other pinna primordia do (8). In both the af uni-tac and the af tl uni-tac genotypes, there is a tendency for the pinnae at position 2 to be simple instead of compound as they are on adult leaves of af and af tl. This suggests that when the uni-tac mutation is added, pinnae at that position are more prone to loss of Uni expression in their primordia than those at position 1.
 A comparison between the adult leaves of these eight genotypes shows that
the mean number of pinna pairs varies. Among
the original Marx lines, WT has the
lowest mean and af tl has the highest.
This correlates well with the relative amount of Uni
mRNA present in the shoot tips (9). Adding
the uni-tac mutation to each of these
genotypes results in a reduction in the number of pinna pairs produced on the
leaves. These reductions are not
uniform across the board. The
reduction of tl uni-tac compared to tl
is more drastic than WT vs. uni-tac and those resulting from addition of this mutation to the af
genotypes are less so. This is
evidence of differential interactions between Uni
and Tl and Af.
These results are consistent with the gradient effects previously
reported for Tl and Af
on pinna form and anatomy in the various hetero-zygotes (6, 17).
For example, Af weakly
represses pinna length of the distal pinnae and strongly represses it in the
proximal pinnae; whereas, Tl strongly
increases pinna length in the distal pinnae and weakly increases it in the
proximal. These gradient
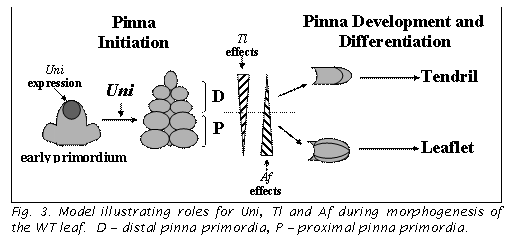
effects are presented in a model (6, Fig. 3).
A comparison between the adult leaves of these eight genotypes shows that
the mean number of pinna pairs varies. Among
the original Marx lines, WT has the
lowest mean and af tl has the highest.
This correlates well with the relative amount of Uni
mRNA present in the shoot tips (9). Adding
the uni-tac mutation to each of these
genotypes results in a reduction in the number of pinna pairs produced on the
leaves. These reductions are not
uniform across the board. The
reduction of tl uni-tac compared to tl
is more drastic than WT vs. uni-tac and those resulting from addition of this mutation to the af
genotypes are less so. This is
evidence of differential interactions between Uni
and Tl and Af.
These results are consistent with the gradient effects previously
reported for Tl and Af
on pinna form and anatomy in the various hetero-zygotes (6, 17).
For example, Af weakly
represses pinna length of the distal pinnae and strongly represses it in the
proximal pinnae; whereas, Tl strongly
increases pinna length in the distal pinnae and weakly increases it in the
proximal. These gradient
effects are presented in a model (6, Fig. 3).
Uni expression is concentrated in the growing leaf tip. Pinna primordia are initiated as long as this expression is maintained, and cease being initiated when it ceases. At this point, the terminal pinna starts to differentiate. This happens precociously in uni and uni-tac mutants. As pinnae are initiated, they determine their position along the leaf axis. Proximal pinnae are more responsive to Af and become leaflets, whereas distal ones are more responsive to Tl and become tendrils. The model illustrates how decreasing the duration of Uni expression in the leaf tip would affect distal pinnae more than proximal pinnae and the tl genotype (i.e. loss of both Tl and Uni function) more than af genotypes.
Seeds for each of these new lines have been deposited in the Marx collection at the USDA Western Regional Plant Introduction Station. The link to these accessions is http://www.ars-grin.gov/cgi-bin/npgs/html/desclist.pl?173. The lines are identified as: uni-tac ¢ W6 27606; af uni-tac ¢ W6 27607; tl uni-tac W6 27608; afila tl uni-tac ¢ W6 27609.
Acknowledgment: I thank Dr. Adam Lukaszewski for a critical reading of the manuscript.
1. Allard, R.W. 1999. Principles of Plant Breeding. John Wiley and Sons, Inc.
2. Busch, A. and Gleissberg, S. 2003. Planta 217: 841-848.
3. DeMason, D.A. and Chawla, R. 2004. Planta 218: 435-448.
4. DeMason, D.A. and Chawla, R. 2004. Int. J. Plant Sci. 165: 707-722.
5. DeMason, D.A. and Schmidt., R.J. 2001. Int. J. Plant Sci. 162: 1033-1051.
6. DeMason, D.A. and Villani, P.J. 2001. Int. J. Plant Sci. 162: 493-511.
7. Goldman, I.L. and Gritton, E.T. 1991. J. Hered. 82: 479-483.
8. Gourlay, C.W., Hofer, J.M.I. and Ellis, T.H.N. 2000. Plant Cell 12: 1279-1294.
9. Hofer, J., Turner, L., Hellens, R., Ambrose, M., Matthews, P., Michael, A. and Ellis, N. 1997. Cur. Biol. 7: 581-587.
10. Lu, B., Villani, P.J., Watson, J.C., DeMason, D.A. and Cooke, T.J. 1996. Int. J. Plant Sci. 28: 138-150.
11. Marx, G.A. 1986. Pisum Newslett. 18: 49-52.
12. Marx, G.A. 1987. Plant Mol. Biol. Rep. 5: 311-335.
13. Marx, G.A. 1974. Pisum Newslett. 6: 60.
14. Sharma, B. 1972. Pisum Newslett. 4: 50.
15. Sharma, B. 1981. Pulse Crops Newslett. 1: 56-57.
16. Villani, P.J. and DeMason, D.A. 1999. Ann. Bot. 83: 117-128.
17.
Villani, P.J. and DeMason, D.A. 1999.