|
Pisum Genetics |
Volume 26 |
1994 |
Research Reports |
pages 26-27 |
Mapping of the chlorophyll mutation vam of the variomaculata-type in linkage group I of pea
|
Rozov, S.M.1, and Gorel', F.L.
|
Institute of Cytology and Genetics Novosibirsk , 630090, Russia |
Chlorophyll mutations are quite common in plants, the phenomenon obviously related to the complexity of the photosynthetic system. In some experiments, chlorophyll mutations constitute 50% of all obtained aberrants (1). The majority of chlorophyll mutations in pea result in forms with the pigment content uniformly changed in all parts of a plant. In contrast, mutants of the so called variomaculata type (1), with leaves bearing larger or smaller spots and sectors of different colours, are quite rare. Nevertheless, more than one hundred cases of induced and spontaneous variomaculata-type mutants have been reported by various authors (1). Only one mutant of this type was assigned to the genetic map. It was obtained and named cr by Wells (4) and then renamed vac-1 by Blixt (1). Wells (4) observed linkage (22.2 ± 5.7%) with gene a in linkage group I.
During the morphological analysis of M2 plants obtained after treating our SGE line with 0.15% EMS, the variomaculata-type mutant line SGE-632 was isolated. The mutant showed single gene recessive inheritance and was assigned gene symbol vam. The leaflets and stipules of this mutant have many irregular shaped spots varying in colour from white, to yellow, yellow-green, green and dark-green. The spots are located very close to each other so that it is impossible to trace the boundaries of a single spot. In general the colouration of a plant may be characterized as "marmoreal". Sometimes leaflets and stipules have white or yellow sectors of a larger size which later become necrotic. Spots and sectors of light colour are situated predominantly along the leaf nerves and edges. Seedlings of the SGE-632 mutant have a yellow-green colouration, while the stem, inflorescences and pods of adult plants have a normal green colour. When grown at low temperatures (12-16°C) in the greenhouse, mutant plants showed spots and necrotic sectors of a predominantly white colouration. The vam mutation does not cause the spotted colouration of cotyledons (line SGE has green cotyledons) as described for some mutants of the variomaculata type.
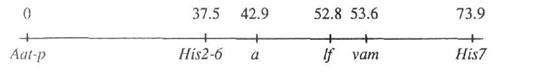
Mutant line SGE-632 (vam) was crossed to the tester-line OK-7, carrying a number of marker genes for linkage group I: Aat-p, a, His2-6, lf, and His7. The results of segregation in F2 are shown in Table 1. Significant (P<0.0001) linkage was detected between gene vam and marker genes a, His2-6, His7, and lf. Linkage with Aat-p was not significant. The map fragment of linkage group I shown below was constructed using the JOINMAP computer program (3) (the Kosambi-function was used, and distances are given in cM):

The recombination fraction between genes vam and a was 14.3±6.1% in our experiment. Therefore we cannot exclude the possiblity that vam and Wells' cr (vac-1) are alleles of one gene. Unfortunately, the vac-1 mutant was lost (2), so it is impossible to carry out a complementation test. The genes vam and vac-1 cause morphologically different patterns: vac-1 produced only light green and dark green spots, observable also in cotyledons, and was expressed only on the early stages of plant growth. The severity of the symptoms appearing on new foliage diminished with the growth of a plant until normal leaves appeared (4). The vam mutation produces spots and sectors of white, yellow and green colours, not affecting cotyledons, and the pattern is stable throughout the life of the plant.
Table 1. Joint segregation data in F2 progeny of cross SGE-632 (vam, A, Aat-pF, His2-6, His7, Lf) x OK7 (Vam, a, Aat-pS, His2-6, His7, lf).
|
Gene pair |
Phase |
Phenotype1 |
Joint Chi-sq. |
Recomb. frac. |
SE |
|||||||||
|
|
|
|
A/B |
A/h |
A/b |
h/B |
h/h |
h/b |
a/B |
a/h |
a/b |
|
|
|
|
vam |
a |
R |
137 |
|
68 |
|
|
|
46 |
|
1 |
18.53* |
14.35 |
6.14 |
|
vam |
Aat-p |
C |
39 |
90 |
29 |
|
|
|
5 |
14 |
10 |
3.97 |
39.08 |
4.29 |
|
vam |
His2-6 |
C |
61 |
127 |
15 |
|
|
|
1 |
18 |
27 |
73.76* |
16.37 |
2.53 |
|
vam |
His7 |
C |
62 |
110 |
28 |
|
|
|
0 |
17 |
28 |
53.42* |
21.24 |
2.89 |
|
vam |
lf |
R |
121 |
|
83 |
|
|
|
47 |
|
0 |
28.57* |
0.11 |
6.31 |
|
a |
Aat-p |
C |
32 |
81 |
26 |
|
|
|
7 |
23 |
18 |
7.26 |
38.15 |
4.26 |
|
a |
His2-6 |
C |
42 |
134 |
6 |
|
|
|
0 |
11 |
56 |
169.76* |
6.43 |
1.60 |
|
a |
His7 |
C |
52 |
100 |
26 |
|
|
|
4 |
27 |
36 |
43.32* |
25.75 |
3.17 |
|
a |
lf |
C |
161 |
|
22 |
|
|
|
7 |
|
61 |
135.18* |
11.35 |
2.15 |
|
Aat-p |
His2-6 |
C |
20 |
21 |
3 |
19 |
72 |
13 |
6 |
20 |
13 |
24,83* |
30.60 |
3.00 |
|
Aat-p |
His7 |
C |
10 |
27 |
7 |
24 |
55 |
24 |
11 |
19 |
9 |
1.82 |
49.34 |
3.66 |
|
lf |
Aat-p |
C |
27 |
73 |
29 |
|
|
|
12 |
31 |
15 |
0.27 |
48.20 |
4.47 |
|
His2-6 |
His7 |
C |
34 |
24 |
4 |
26 |
84 |
32 |
2 |
19 |
20 |
54.00* |
27.30 |
2.46 |
|
lf |
His2-6 |
C |
40 |
118 |
9 |
|
|
|
2 |
27 |
53 |
106.06* |
14.91 |
2.42 |
|
lf |
His7 |
C |
52 |
93 |
19 |
|
|
|
4 |
34 |
43 |
56.17* |
23.99 |
3.07 |
1A,a - first gene; B,b - second gene; h - heterozygous.
1. Both genes are dominant: capital letter stands for dominant allele.
2. Second gene is codominant: capital A stands for allele of the first gene and capital B for an allele of the second gene, being in coupling with A.
3. Both genes are codominant: capital letter stands for an allele of the first parent.
* Probability < 0.0001.
Acknowledgements. This work was partially supported by the Russian State Programs "Fundamental Investigations" and "Frontiers in Genetics".
Blixt, S. 1972. Agri Hort. Genet. 30:1-293.
Blixt, S. 1977. Pisum Newsl., 9, Supplement.
Stam, P. 1993. The Plant Journal 5:739-744.
Wells, D.G. 1951. J. Genet. 50:230-234.