|
Pisum Genetics |
Volume 26 |
1994 |
Research Reports |
pages 18-20 |
Phytohormones in a chlorophyll-deficient pea mutant of the "xantha"-type
|
Kof, E.M.*, Gostimski , S.A. ** and Kefeli, V.I.*
|
*K.A. Timiryazev Institute of Plant Physiology Russian Academy of Sciences, Botanicheskaya Str. 35 Moscow , 127276 Russia **Department of Genetics, Moscow State University Moscow , 119899 Russia |
The question of the role of the photosynthetically active chloroplast and of light in morphogenesis and phytohormone synthesis is of fundamental importance. The chloroplast is one of the main sites of the synthesis or the action of the phytohormones abscisic acid (ABA), indole-acetic acid (IAA), and cytokinins, which take part in the regulation of growth, chloroplast biogenesis and greening, and stomatal movement (1, 2, 4, 5). The modern approach to the resolution of this question is to use chlorophyll-deficient mutants to uncouple the light effects on hormonal synthesis and growth from those on photosynthesis.
Materials and Methods
The pea mutant of "xantha"-type used in our experiments was obtained after treating seeds of cv Svoboda by NMU. The mutant yellow phenotype of line N14 is caused by a nuclear recessive mutation. The plastids of this mutant are smaller than chloroplasts of the wild-type green form. Their lamellar structure consists of infrequent vesicles and they are unable to carry out photosynthesis (3). Mutant plants die before producing seeds and the allele is maintained through the heterozygotes. Seeds homozygous for the mutant allele can be distinguished by their light-yellow colour compared with the light-green colour of seeds bearing the wild-type allele. Cv Svoboda was used as the wild-type in all experiments.
ABA level was determined by a GC (gas chromatograph) equipped with an electron capture detector. IAA content was estimated by HPLC (PYE unicam PU 4002 liquid chromatograph). Extraction and determination of cytokinins was performed using the Amaranthus-bioassay method of van Staden and Carmy (9). The content of chloroplast pigments was calculated by the method of Wettstein (8). Dry weight was determined from duplicate samples not used for extraction. The fresh-weight to dry-weight ratio was 7.1:1 and 13.3:1 for light-grown and dark-grown plants, respectively. Plant samples for phytohormone analysis were placed in liquid nitrogen prior to the extraction procedures. The upper part of the plant included the shoot only. The cotyledons were discarded. The data are based on the combined results of three experiments. Eight to ten plants were harvested for each treatment and five analyses were used to provide each datum. The plants were raised in a growth chamber at a temperature of 24-26�C. Light-grown plants received a 12 h photoperiod under LB-65 lamps providing an intensity of 10,000 lux. The plants were grown in 0.5 L glass containers filled with water which was changed daily. The 8-day-old seedlings were harvested at the stage when they had 6-7 nodes (4-5 normally developed leaves) and before the onset of retarded growth in the mutant plants. Standard deviation and standard error were calculated according to Student's criteria. Differences are discussed only at P<0.05.
Results
Dark-grown plants of wild-type and mutant lines had reduced leaves and a closed apical hook. They did not differ in their stem and root length and weight. Likewise stem growth and leaf morphogenesis of light-grown wild-type and mutant seedlings were similar. Their leaves developed normally and elongation of the stem was depressed compared with dark-grown plants. However, light-grown mutant plants, like dark-grown wild-type and mutant seedlings, had no chlorophylls. The carotenoid content of light grown mutant plants was about 12% of the level in the wild-type green line. Carotenoids were practically absent in the dark-grown seedlings of both lines (7).
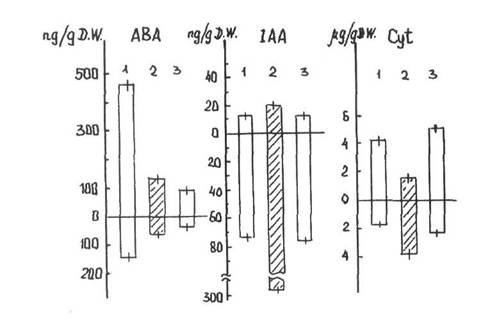
The content of ABA in the upper part of light-grown wild-type plants was much higher than in the upper part of light-grown mutant plants and of dark-grown seedlings of the wild-type (Fig. 1). The ABA content was 3-4 times lower in the roots of all forms in comparison with their shoots. The ABA content was 2-3 times as high in the roots of light-grown wild-type plants as in the roots of the chlorophyll-deficient light-grown mutant plants and dark-grown seedlings. It is possible that the decreased ABA content in mutant plants is related to decreased synthesis of mevalonic acid, a precursor common to ABA and carotenoids, or with a change of mevalonic acid metabolism to the carotenoid C40 pathway. In the latter case our results could be taken as indirect evidence of the carotenoid pathway of ABA synthesis, as proposed by Neill et al. (6) and other researchers.
The light-grown wild-type and mutant plants (both their upper part and roots) did not differ in the content of IAA (Fig. 1). Moreover, the IAA content was 3-4 times higher in the roots than in the upper part of the seedlings. The level of IAA was higher in dark-grown seedlings than in light-grown plants, especially in their roots where the difference exceeded 3-fold. Thus the blockage of chloroplastogenesis did not change IAA level but IAA level was influenced by whether the plants were grown in light or darkness.

Fig. 1. The content of ABA, IAA and cytokinins in light-grown wild-type (1) and mutant (3) plants, and in dark-grown wild-type plants (2). A - upper part, B - roots. The bar lines indicate one standard deviation.
The level and distribution of cytokinins was approximately the same in the light-grown wild-type and mutant plants (Fig. 1). In contrast to IAA levels in light-grown plants, the level of cytokinins was higher in the upper part of the plant than in the roots. However, in dark-grown seedlings the level of cytokinins was higher in the roots than the shoot. The content of cytokinins decreased in the roots and increased in the upper part of the seedlings after 4 hours exposure to light It is possible that cytokinin transport from the roots to the upper part of the plant was depressed in the darkness. After exposure to light, cytokinin transport appeared equally intensive in both the wild-type green and the mutant yellow lines. However, in the mutant plants the stimulating effects of cytokinins on greening could not be realised because of the genetic block in chloroplast biogenesis.
These results show that growth and morphogenesis, and the content and distribution of plant growth regulators, depend on light. However, the level of IAA and cytokinins is not related to chloroplastogenesis, greening and photosynthesis. The level of ABA depends both on light and on the presence of green photosynthetically active chloroplasts. The blockage of greening and photosynthesis in the chlorophyll-deficient mutant leads to a substantial decrease in ABA content. Naturally, these data are correct only when storage substrates are available from the cotyledons.
Chen, CM. 1981. In: Metabolism
and Molecular Activities of Cytokinins. Eds. J.
Gueen and C. Peaud-Lenoel. Springer-Verlag, N.Y. pp. 34-43.
Fregeau, J.A. and Wightman, F. 1983. Plant Sci. Letters. 32:23-34.
Gostimski,
S.A. ,
Karvovskaja, K.A., Sineshchekow, V.A. and Beljaeva, O.B. 1982.
Genetica (Russia). 18:124-132.
Kaizer, G., Weiler, E.W. and Hartung, W. 1985. J. Plant Physiol. 11:237-245.
Loveys, B.R. 1987. Physiol. Plant. 40:6-10.
Neill, S.J., Horgan, R. and Walton, D.C. 1984. In: Biosynthesis and Metabolism of
Hormones. Eds. A. Crozier and J.R. Hillman. Cambridge Univ. Press, pp. 43-70.
Polyakov, A.S., Kof, E.M. and Gostimski,
S.A. 1985. Biologia Plantarum. 27:139-
144.
Shlik, A.A. 1971. In: Biochemical
Methods in Plant Physiology. Ed. O.A. Pavlinova.
Nauka, Moscow, pp. 154-170.
Staden van J. and Carmy, A. 1992. Physiol. Plant. 55:39-44.