|
Pisum Genetics |
Volume 23 |
1991 |
Research Reports |
pages 19-25 |
Differences in flowering behaviour between cultivar Ranny Zeleny and regenerant lines developed from callus cultures are attributable to variation at the Lf and Sn loci.
|
Murfet, I.C. and Ezhova, T.A. |
Dept of Plant Science, Univ. of Tas. Hobart Tas. 7001, Australia Dept of Genetics, Moscow State Univ., Moscow 119899, Russia |
Ezhova et al (3) reported identifying from callus cultures of cv Ranny Zeleny several regenerant lines which differed from the initial line in their flowering behaviour. One group of regenerants typified by line R1 flowered at a higher node than Ranny Zeleny while a second group typified by line R9 flowered at a lower node than Ranny Zeleny. Both classes of regenerants were more vigorous than the initial line. Crosses among the regenerants and between the initial line and the regenerants indicated that the differences in flowering behaviour were determined by changes in the allelic state for two flowering genes. Using symbols A/a and B/b for illustrative purposes Ranny Zeleny could be symbolised as genotype "AA bb", regenerant R1 (high flowering node) as "AA BB" and regenerant R9 (low flowering node) as "aa BB".
We report here the results of genetic tests and physiological comparisons of Ranny Zeleny and regenerant lines against Hobart lines of known flowering genotype. The studies, carried out at Hobart and Moscow, show that the locus temporarily symbolised "A" is in fact the one symbolised Lf by White (16) and that the locus temporarily shown as "B" is the one symbolised as Sn by Barber (1) and Murfet (5).
Materials and Methods
The Hobart lines used in the study represent four previously defined flowering classes (see 5, 10). They include lines 59 (lf E sn Dne hr) and 73 (Lf E sn Dne hr) representing the day neutral (DN) class, line 60 (lf E Sn Dne hr) representing the early photoperiodic (EI) class, line 24 (Lf e Sn Dne hr) representing the late photoperiodic class with a quantitative response to photoperiod (class L), and line 63 (lf e Sn Dne Hr) representing the late photoperiodic class with a high response to photoperiod (class LHR). Cultivar Kaliski (Lf E Sn Dne hr) is also used to represent the late phutoperiodic class in Table 1. Moscow lines include Ranny Zeleny and regenerants R9 (low flowering node) and R1 and R11 (high flowering node). Two plants each of Ranny Zeleny, R9 and Rl1 were brought through quarantine at Hobart. The material labelled Ranny Zeleny was given the Hobart accession number L221. The two plants in fact had the same phenotype as R11 but in subsequent generations segregants with a true Ranny Zeleny phenotype appeared as a single gene recessive trait. Subsequent tests showed the accession was heterogeneous for alleles Sn and sn, and the late (Sn) and day neutral (sn) forms of the accession were designated lines 221+ and 221-, respectively.
At Moscow plants were grown in the field during May and June when the photoperiod ranged from 15.5-17.5 h. At Hobart plants were grown in the phytotron in 14 cm slim line pots using a growth medium of vermiculite and dolerite chips topped with a peat/sand potting mixture. Nutrient (Hoaglands #1) was supplied once a week. Plants in short day conditions received 8 h of daylight and 16 h of darkness. Plants in long day conditions received 8 h daylight and 16 h incandescent light (3 mmol m-2 s-1).
Table 1. Means of several reproductive traits for pea lines grown in the phytotron at Hobart under a 24 h (8 h daylight + 16 h incandescent light at 3 mmol m-2 s-1) or an 8 h (8 h daylight + 16 dark) photoperiod. Temperature day 23-25�C, night 16 �C.
|
Line (Genotype) Phenotype |
Photoperiod |
FI |
FD |
FP |
FT |
TN |
RN |
Pods |
Seeds |
N |
|
59 (lf E sn Dne hr) |
24 |
9.0 |
9.0 |
9.0 |
32.8 |
11.0 |
3.0 |
3.0 |
20.0 |
4 |
|
Early, day neutral |
8 |
9.3 |
9.3 |
10.0 |
35.8 |
13.8 |
5.5 |
4.0 |
22.5 |
4 |
|
73 (Lf E sn Dne hr) |
24 |
12.7 |
12.7 |
12.7 |
36.7 |
17.0 |
5.3 |
3.7 |
21.3 |
3 |
|
Day neutral |
8 |
12.0 |
12.0 |
12.0 |
36.7 |
17.3 |
6.3 |
7.3 |
36.0 |
3 |
|
60 (lf E Sn Dne hr) |
24 |
11.0 |
11.0 |
11.0 |
36.3 |
15.3 |
5.3 |
6.0 |
31.5 |
4 |
|
Early photoperiodic |
8 |
10.7 |
12.3 |
12.7 |
49.7 |
37.7 |
28.0 |
33.5 |
106.0 |
3 |
|
Kaliski (Lf E Sri Dne hr) |
24 |
15.0 |
15.0 |
15.0 |
40.8 |
19.5 |
5.5 |
6.3 |
28.3 |
4 |
|
Late photoperiodic |
8 |
22.5 |
22.5 |
22.5 |
59.0 |
36.5 |
15.0 |
24.0 |
74.3 |
4 |
|
221- |
24 |
12.6 |
12.6 |
12.6 |
34.2 |
14.5 |
2.9 |
1.9 |
7.6 |
12 |
|
Day neutral |
8 |
12.7 |
12.7 |
12.7 |
35.3 |
17.0 |
5.3 |
3.2 |
13.1 |
12 |
|
R9 |
24 |
11.8 |
11.8 |
11.8 |
36.3 |
14.0 |
3.3 |
2.3 |
9.5 |
4 |
|
Early photoperiodic |
8 |
12.0 |
17.7 |
17.7 |
54.3 |
30.0 |
19.0 |
13.7 |
56.7 |
3 |
|
221+ |
24 |
16.5 |
16.5 |
16.5 |
41.8 |
19.5 |
4.0 |
2.3 |
11.7 |
4 |
|
Late photoperiodic |
8 |
25.7 |
25.7 |
25.7 |
60.3 |
33.7 |
9.0 |
10.3 |
52.0 |
3 |
|
R11 |
24 |
16.3 |
16.3 |
16.3 |
42.3 |
19.3 |
4.0 |
2.5 |
12.8 |
4 |
|
Late photoperiodic |
8 |
25.8 |
25.8 |
26.0 |
61.8 |
35.5 |
10.8 |
10.3 |
52.5 |
4 |
FI node of flower initiation counting from the first scale leaf as node 1.
FD node of first developed flower.
FP node of first pod.
FT flowering time; days from sowing to firsr open flower.
TN total number of nodes (with expanded leaves) on main stem.
RN number of reproductive nodes on main stem.
Pods and Seeds (number per plant).
N number of plants.
Night temperature was 16�C and day temperature was generally in the range of 20-25�C. The data in Table 1 are based mostly on a small sample size and they come from two separate studies (lines 59, 60, 73 and Kaliski were sown Dec. 23 in phytotron 2 and 221-, R9, 221+ and R11l were sown Dec. 29 in phytotron 1). Nevertheless, the data satisfy the primary aim to determine the phenotypic classification of the Moscow lines. Plants in Fig. 1 were sown Feb. 24 and in Fig. 2 Mar. 23. Node counts commenced from the first scale leaf as node 1. Flowering node data were obtained only from primary stems. At Hobart, laterals were regularly excised. Further details on traits recorded are given at the foot of Table 1.
Results and Discussion
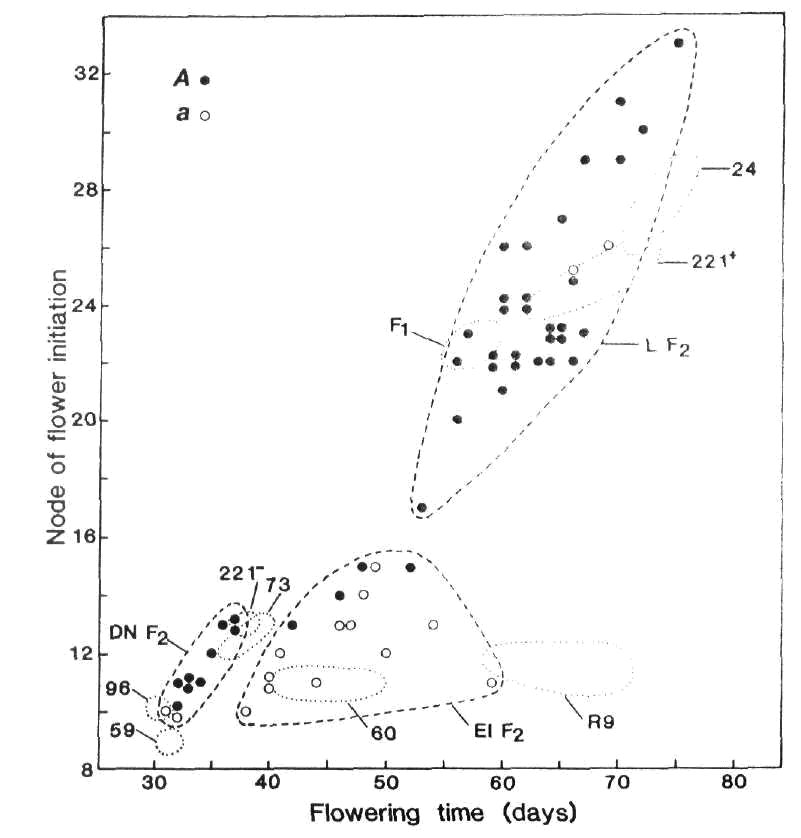
The flowering behaviour and phenotypic classification of the Moscow lines is readily apparent from the data in Table 1. Lines 221+ and R11 showed a quantitative increase of 9-10 nodes and 18-20 days in flowering node and time, respectively, in the short day conditions. These characteristics closely match those of plants belonging to the late photoperiodic class represented here by Kaliski (Table 1) and the type line for the L class, line 24 (Fig. 1). Regenerant R9 behaved in a manner typical of the early photoperiodic class. Node of flower initiation was not influenced by photoperiod but the number of days to first open flower increased by 18 days in the short day photoperiod due mostly to abortion of the lower flower buds (Table 1). R9 also showed a 5 to 6-fold increase in the number of reproductive nodes and seed yield in short days. These characteristics are clearly similar to those displayed by the type line for the early photoperiodic class, line 60 (Table 1, Fig. 1). Finally, L221- (Ranny Zeleny) displayed characteristics typical of the day neutral class and its behaviour closely matched that of line 73 (Table 1, Figs 1 and 2).

Fig. 1. Node of flower
initiation plotted against days to first open flower for F2
plants of cross 73 (A
Lf E sn Dne hr) x
R9 (a lf E Sn Dne hr).
The boundaries of the three phenotypic
classes late (L), early photoperiodic (EI) and day neutral (DN) are indicated by the broken lines (![]() )
and the flower colour is indicated by open or closed circles (○ white,
● red). The plots for the parental lines 73 and R9, the F1 and reference lines 59 (lf
E sn Dne hr), 96 (lf
e sn Dne hr), 221- (Lf E sn Dne hr), 60 (lf E Sn Dne hr), 221+
(Lf E Sn Dne
hr) and 24 (Lf e Sn Dne hr)
fall within the dotted (
)
and the flower colour is indicated by open or closed circles (○ white,
● red). The plots for the parental lines 73 and R9, the F1 and reference lines 59 (lf
E sn Dne hr), 96 (lf
e sn Dne hr), 221- (Lf E sn Dne hr), 60 (lf E Sn Dne hr), 221+
(Lf E Sn Dne
hr) and 24 (Lf e Sn Dne hr)
fall within the dotted (![]() )
boundaries. Photoperiod 8 h.
)
boundaries. Photoperiod 8 h.
The phenotypic data in Table 1 are sufficient by themselves to predict the genotypes with confidence given that the Moscow lines all derive from the same background. The flowering node of 11-12 and early photoperiodic habit of line R9 implies a genotype of lf E Sn Dne. The fact that line R11 is a late photoperiodic type with a limited quantitative response to photoperiod implies genotype Sn Dne hr. [Gene Hr confers a high response to photoperiod (8)]. The flowering node of 12 to 13 and day neutral habit of L221- (Ranny Zeleny) is indicative of genotype of Lf sn. The predicted genotypes are therefore: - Lf E sn Dne hr (L221-, Ranny Zeleny), Lf E Sn Dne hr (L221+, R11) and lf E Sn Dne hr (R9). These genotypes would also account for the results obtained (3) from crosses among the Moscow lines. These predicted genotypes have now been verified by genetic tests against Hobart lines of known genotype.
The F1 of cross 221- x 73 (Lf E sn Dne hr) had very similar characteristics to the two parental lines (Fig. 2) indicating that line 221- (Ranny Zeleny) has genotype sn. If the day neutral habit of line 221- was due to dne or a mutation at some currently unidentified locus then the F1 would be expected to belong to the late photoperiodic class.

Fig. 2. Node of flower initiation plotted against days to first open flower for the F1 of cross 73 x 221- (▲) and parental lines 73 (○) and 221- (□).
The early photoperiodic regenerant R9 (predicted genotype lf E Sn Dne hr) was crossed with line 73 (Lf E sn Dne hr) and F1, F2 and F3 generations raised in an 8 h photoperiod. As expected, the F1 was phenotypically late; it flowered at nodes 23-24 compared with nodes 11-13 for the two parents (Fig. 1). The F2 segregated into three classes with observed numbers of 32 late photoperiodic, 16 early photoperiodic and 11 day neutral plants (Fig. 1). The expected dihybrid ratio for segregation of Lf-lf and Sn-sn on a E Dne hr background is 9 late photoperiodic : 3 early photoperiodic : 4 day neutral with expected numbers of 33, 11 and 15, respectively. The observed numbers are in accord with expected numbers (c2 = 3.37, P > 0.1). In fact the observed numbers are even better than they first appear for two reasons. First, the Sn -sn pair of alleles consistently segregates closer to 4:1 than 3:1 (6). Second, F3 data showed that the four red flowered, early photoperiodic F2 segregates (Fig. 1) all had genotype Lflf Sn- since the majority of their F3 offspring flowered in the late class. In contrast, the white flowered, early photoperiodic F2 segregates flowering at nodes 14 and 15 produced no late segregates in F3 consistent with their expected genotype of lflfSn-. The non-random distribution of flower colour in the F2 provides further evidence that the regenerant R9 has genotype lf. The Lf locus is on chromosome 1 about 10 centimorgans from the A locus (6, 9, 18) and cross 73 (A Lf) x R9 (a lf) is in the coupling phase. Among photoperiodic F2 segregates, all genuine early segregates were white flowered (aa) and the vast majority of late segregates were red flowered (30 red A-, 2 white aa). The joint segregation Chi-square for A and Lf on the Sn background is 38.9, P < 0.001, recombination fraction < 6%. The effect of segregation for Lf-lf is also apparent among day neutral (sn) segregates because the two white flowered (aa) plants flowered at node 10 while most red flowered (A-) plants flowered at nodes 11 to 13.
Evidence that the late photoperiodic regenerant line R1 carries Lf was obtained from the F2 of a cross between R1 and Hobart line 63 (A lf e Sn Dne Hr) raised in the field at Moscow in May-June of 1990. If line R1 has genotype a Lf E Sn Dne hr the cross is trihybrid for the flowering genes. All offspring should be photoperiodic (Sn Dne always present). The theoretical segregation ratio is 39 (late, high response; Lf E Hr, Lf e Hr, lf e Hr) : 13 (late; Lf E hr, Lf e hr, lf e hr) : 9 (early photoperiodic like Marx G2; lf E Hr): 3 (early photoperiodic like line 60; lf E hr). However, the long day photoperiod at Moscow would largely eliminate the effect of Hr and the ratio would reduce to the form 13 late : 3 early. Moreover, the low temperatures at Moscow during germination and early growth of the seedlings would increase the frequency of low flowering node off-types (impenetrant lates) among segregates with genotypes lf e Sn Dne hr or lf e Sn Dne Hr. [The occurrence of some phenotypically early plants in these genotypes is well known (5, 6, 8) and the behaviour is explained by the threshold nature of flower initiation, the relative ease with which an lf apex is triggered and the reduction in Sn Dne activity at low temperatures (7, 13, 17)]. The expected frequency of F2 segregates classifying as phenotypically early flowering will therefore increase to some value between three sixteenths and one quarter. The phenomenon of impenetrance in the lf e Sn Dne genotypes was exemplified by the fact that some of the genetically pure line 63 (lf e Sn Dne Hr) control plants flowered as early as node 11 (observed L63 flowering node range 11-17; most common values 15 and 16).
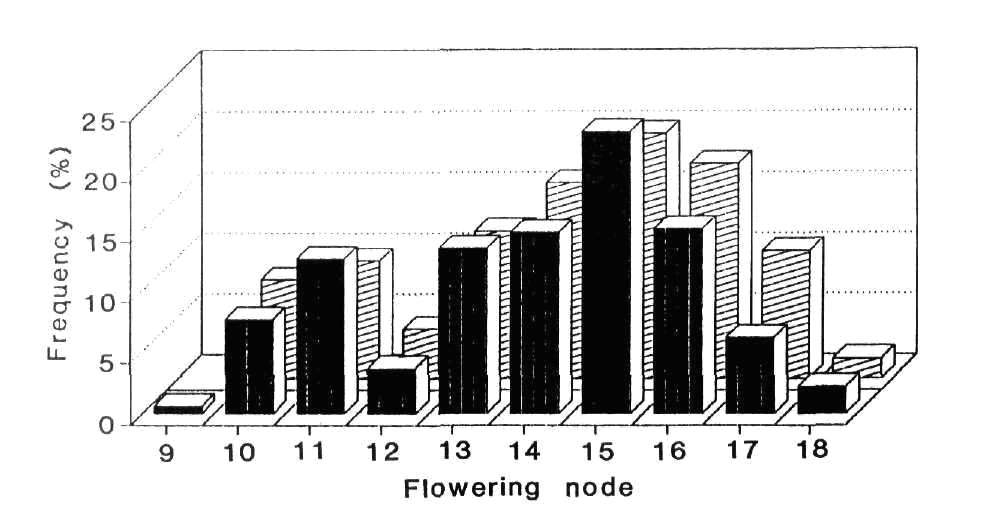
Fig. 3. Frequency distribution for node of flower
initiation in the F2 of cross 63 x R1 grown in the field at Moscow
May-June 1990. ![]() Le (323 plants)
Le (323 plants) ![]() le
(124 plants).
le
(124 plants).
The flowering node frequency distribution for the L63 x R1 F2 is shown in Fig. 3. Cutting the continuous but clearly bimodal distribution between nodes 12 and 13 gives observed numbers of 340 late and 106 early segregates. These numbers are in good agreement with a 3:1 ratio (c2 = 0.36, P > 0.5) but they differ significantly from a 13:3 ratio (c2 = 7.39, P < 0.01). As explained above, the phenotypically early plants flowering at nodes 9-12 almost certainly include some genetically late segregates. As predicted, the observed number of 106 early segregates lies between the numbers expected with a 13:3 (84 early) and a 3:1 (112 early) ratio. We believe therefore that the results fit the genetic model proposed. Further confirmation is provided by the joint segregation data for high versus low flowering node and red (A) versus white (a) flower colour. The observed numbers were 230 high, red, 110 high, white; 106 low, red; 0 low, white. The joint segregation Chi-square obtained using a 2 x 2 contingency table (c2 = 45.2; P < 0.001) shows a highly significant deviation from independent assortment. The cross L63 (A lf) x R1 (a Lf) is in the repulsion phase for A and Lf but because some lf e segregates very likely occurred in both the high and low flowering node classes the joint segregation data as presented do not represent in the strict sense the joint segregation data for Lf-lf and A-a. Nevertheless, the results clearly indicate that R1 carries Lf and that the Lf-lf segregation is the principal cause of the segregation into the high and low flowering node classes.
The flowering node distributions for the tall (Le) and dwarf (le) segregates in the L63 x R1 F2 were fairly similar (Fig. 3). The le allele is known to quantitatively increase flowering time (4, 15) by slowing the rate of leaf appearance (14).
We conclude that Ranny Zeleny and line 221- have genotype Lf E sn Dne hr, that line R9 is lf E Sn Dne hr and that lines R1, R11 and 221+ are Lf E Sn Dne hr. This conclusion was also supported by the results of other crosses penurmed at Moscow and Hobart but not documented here.
The question of whether late flowering regenerants like R1 and R11 arose by back mutation of sn to Sn in the callus culture or as a result of heterogeneity in the original Ranny Zeleny material used in 1982 to start the long term callus cultures cannot wholly be resolved. Clearly, heterogeneity for Sn and sn can occur in cultivar Ranny Zeleny, as witnessed by the two forms of line 221. On the other hand, the effect of Sn is readily apparent in Moscow conditions and heterogeneity at this locus would be obvious phenotypically. To get from Ranny Zeleny to line R9 would require two changes, namely sn to Sn and Lf to lf, and this order of events was suggested earlier (3). The odds against consecutive mutations in two flowering genes are high and if the occurrence of Sn regenerants does genuinely result from mutation in the callus cells this would be the first documented case of mutation, either forward or back, at the Sn locus. In contrast, the Lf locus seems to be a mutation hot spot since the great majority of tested and identified flowering mutations in pea have proved to be forward mutations at this locus (see 10, 11). Nevertheless, it should be noted that major mutations at the Lf locus would be phenotypically obvious in all normally encountered genetic backgrounds and under all normally used environmental conditions. In contrast, because of epistasis, mutation of E to e would only be detected in a restricted set of backgrounds, e.g. lf Sn Dne. Likewise, mutations at the Hr locus would not be detected in a sn background and they may even escape detection in a Sn Dne background under long day field conditions, e.g. the Hr-hr segregation is not apparent in the R1 x L63 F2 (Fig. 3).
The Sn regenerants (both lf and Lf lines) are more vigorous than Ranny Zeleny (3). The relative ability of cell lines of genotypes Sn and sn to compete in callus culture is a matter of some interest. The Sn Dne product is graft-transmissible and hormone-like, it delays flower initiation, and (by analogy with sweet pea) it directs assimilate flow toward vegetative growth (2, 7, 12, 16). Callus culture studies using Ranny Zeleny and its isoline regenerants may shed further light on the nature and action this scientifically interesting and practically important gene system.
Acknowledgement. We thank the Australian Research Council for financial support of the work at Hobart.
Barber, H.N. 1959. Heredity 13:33-60.
Beveridge, C.A., Ross, J.J. and Murfet, I.C. 1992. J. Exp. Bot. (in press).
Ezhova, T.A., Bagrova, A.M. and Gostimski, S.A. 1990. PNL 22:15-17.
Marx, G.A. 1975. PNL 7:30-31.
Murfet, I.C. 1971. Heredity 26:243-257.
Murfet, I.C. 1971. Heredity 27:93-110.
Murfet, I.C. 1971. Aust. J. Biol. Sci 24:1089-1101.
Murfet, I.C. 1973. Heredity 31:157-164.
Murfet, I.C 1975. Heredity 35:85-98.
Murfet, I.C. 1985. In Handbook or Flowering, Ed
A.H. Halevy, Vol. IV,
CRC Press, Boca Raton, Florida, pp 97-126.
Murfet, I.C. Pisum Genetics 23:16-18.
Murfet, I.C. and Reid, J.B. 1973. Aust. J. Biol. Sci. 26:675-677.
Murfet, I.C. and Reid, J.B. 1974. Z. Pflanzenphysiol. 71:323-331.
Murfet, I.C. and Reid, J.B. 1987. J. Plant Physiol. 127:23-29.
Rasmusson, J. 1935. Hereditas 20:161-180.
Paton, D.M. and Barber, H.N. 1955. Aust. J Biol. Sci. 8:231-240.
Reid, J.B. and Murfet, I.C. 1980 Ann Bot. 45:583-586.
White, O.E. 1917. Proc. Amer. Phil. Soc. 56:487-589.