|
PNL |
Volume 22 |
1990 |
RESEARCH REPORTS |
pages 15-17 |
THE MODE OF INHERITANCE OF SOMACLONAL VARIATIONS IN PEA DEALING WITH FLOWERING BEHAVIOUR
|
Ezhova T.A., A.M. Bagrova and S.A. Gostimski |
Genetics Dept., Moscow State Univ. Moscow 119899, USSR |
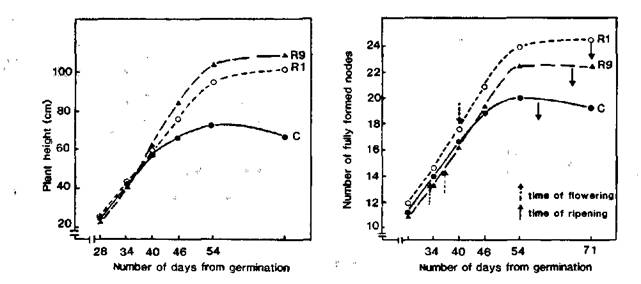
As reported earlier, we have found somaclonal variations among regenerants arising from the longterm callus cultures of the pea cultivar Ranny Zeleny. The somaclonal variations related to the position of first floral node (FN = node of flower initiation counting from the first scale leaf as node 1) and flowering time (FT = days to first open flower) and were inherited by subsequent seed generations (1, 2). As a result, we have obtained 43 regenerant lines flowering at nodes 10-11 (counting from the first scale leaf as node 1) and 7 lines flowering at nodes 14-16. The initial cultivar, Ranny Zeleny, generally flowers at node 12. All these regenerant lines exhibited an increase in FT (2-7 days later in lines with a low FN and 5-14 days later in lines with a high FN) and a more vigorous habit (i.e. taller and thicker stems, longer size of leaves, pods, seeds etc.) in comparison with the initial variety. In the early stages of growth, the growth rate was similar in the control and regenerant plants (Fig. 1 a, b) but the control plants ceased growth sooner and ripened more rapidly than the regenerant plants. Thus the control plants had a shorter vegetative phase, a shorter reproductive phase and a shorter overall life span than the regenerant plants.
Crosses between regenerant and control plants were made to study the mode of inheritance of somaclonal changes. When regenerant plants belonging to different lines but exhibiting the same phenotype were crossed among themselves, the F1 hybrids showed a FN pattern identical to the parents. Different regenerant lines with the same phenotype therefore have identical mutations. In crosses between regenerants with a high FN and those with low FN and in the crosses of regenerants with control plants, the F1 hybrids exhibited dominance of the higher FN arrangement. Reciprocal crosses gave the same results; therefore the mutations in the regenerants are mutations of nuclear genes.

Fig. 1. Growth of the pea cultivar Ranny Zeleny (C) and regenerants with high (line R1) and low (line R9) flowering node.
Table 1. Distribution of flowering node (FN) in the initial line Ranny Zeleny, and regenerant lines R1 and R9, and in the F1 and F2 generations of crosses R9 x R1, R1 x Ranny Zeleny and R9 x Ranny Zeleny. The results of reciprocal crosses were summed. The plants were grown in the field in the Moscow region during May and June when the photoperiod ranged from 15.5-17.5 h.
|
|
Flowering node |
|||||||
|
Line Year |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
|
Ranny 1988 |
|
|
11 |
35 |
5 |
|
|
|
|
Zeleny 1989 |
|
|
2 |
144 |
73 |
7 |
|
|
|
R9 1988 |
2 |
62 |
28 |
|
|
|
|
|
|
1989 |
2 |
64 |
114 |
10 |
|
|
|
|
|
R1 1988 |
|
|
|
|
2 |
24 |
4 |
|
|
1989 |
|
|
|
|
|
4 |
68 |
30 |
|
F1 1988+1989 |
|
|
|
|
|
|
|
|
|
R9 x R1 |
|
|
|
|
23 |
40 |
5 |
|
|
R1 x Control |
|
|
|
|
|
18 |
37 |
1 |
|
R9 x Control |
|
|
|
1 |
12 |
19 |
1 |
|
|
F2 1989 |
|
|
|
|
|
|
|
|
|
R9 x R1 |
6 |
48 |
42 |
2 |
37 |
129 |
81 |
9 |
|
R1 x Control |
|
|
16 |
47 |
5 |
46 |
176 |
37 |
|
R9 x Control |
10 |
74 |
57 |
29 |
68 |
92 |
72 |
6 |
In the F2 from crosses of high FN regenerants with control plants and with low FN regenerants, the FN distribution was markedly bimodal. The observed numbers in the two near-distinct groups corresponded to the monohybrid ratio 3:1 with dominance of the higher FN arrangement (Table 1). In contrast, in the F2 generations obtained from the crosses between low FN regenerants with control plants the FN distribution differed distinctly from that expected from a monohybrid cross. Three features of the latter F2 distributions were noteworthy: 1) there emerged some plants with a higher FN than either parent, 2) there was a very low number of plants flowering at the 12th node (the modal node in the control plants), and 3) the ratio of high to low FN plants differed considerably from a normal 3:1 ratio (Table 1).
From these results we have assumed that changes in flowering behaviour in our regenerants were caused by mutations in two genes, which complemented when represented by dominant alleles (recessive epistasis also may be assumed). For the purposes of formulating a model we will use symbols A-a_ and B-b to represent the two pairs of alleles (unique symbols will be assigned if allelism tests show either locus is novel). The genotypes may be represented in the following way:
aaBB - variety Ranny Zeleny
AABB - regenerants with a high FN
AAbb - regenerants with a low FN,
where: A is a dominant mutation that prolongs the vegetative period and at the same time causes a delay in the FT. In combination with the dominant allele of gene B, the allele A increases the FN.
b is a recessive mutation reducing the FN (1-2 node reduction).
In such a case the expected segregation in the F2 from a cross of control plants with low FN regenerants should be:
4/16 plants with a low FN (A-bb + aabb)
3/16 plants like the controls (aaB-)
9/16 plants with a high FN (A-B-) The observed F2 segregation was in accord with such an 4:3:9 ratio and F3 analysis confirmed our assumption. In the offspring of F2 plants with a high FN we found some pure breeding families (AABB) and other families which segregated in various ways, e.g. into high FN and low FN plants (AABb) or into high FN and control-type plants (AaBB). All the offspring of plants with a low FN (A-bb and aabb) flowered at nodes 9-11 but a difference was observed in FT. Some families were identified which did not differ from the control plants in FT but they flowered at nodes 9-11 (aabb).
We suppose that the mutations which we found while studying regenerants have arisen in callus cells and then were multiplied during the time of callus cultivation but heterogeneity in the starting material cannot be excluded. (The multiplication of mutations takes place only in callus, but not at the stage of shooting, because to avoid getting adventitious shoots from the same meristematic zones, the shoots were taken from flasks along with the pieces of callus from which they had been derived). Long-term calluses of Ranny Zeleny were obtained in three independent experiments (held in February, August and October 1982) and in all these experiments, after the different time of cultivation, regenerants were obtained with a prolonged vegetative period and higher FN (dominant mutation of gene A). Obviously the callus cells with this mutation have a selective advantage over the cells of the initial line. Most probably this mutation changes the hormonal status of cells and permits them to cope better in the culture conditions.
The regenerants with low FN (A-bb) were obtained in two of these experiments (from calluses with a very good regeneration capacity). Apparently, the recessive mutation to allele b has arisen (and/or became homozygous) in a cell which already had the dominant mutation to allele A.
Later on we hope to establish the relationship between these mutations and already known mutations which influence the flowering process and the duration of the vegetative period.
In conclusion we want to note that callus cells, as well as regenerated plants, can be heterozygous in locus A. The evidence comes from observation of heterogeneity in the FN in some regenerant lines with high FN and heterogenity in the segregation for FN in some F2 families. The appearance of recessive homozygous segregants (aa) in the offspring of heterozygous plants may explain a visible decrease in certain characters that we have found during multiplication of regenerants. In the first years of examination of regenerants we have observed not only the increase in the growth habit, but also changes in leaf shape and size, an increase in the size of the pods and seeds, and a darker green colour of the leaves (2). Later on these changes became less evident.
Ezhova, T.A., A.M. Bagrova and S.A. Gostimski. 1985. PNL 17:8-9.
Ezhova, T.A., A.M. Bagrova, G.A. Hartina and S.A. Gostimski. 1989. Genetica (Russ.) 25(5):878-885.