Alcohol dehydrogenase (ADH) isozymes have been intensively investi-
gated in several crop species (1, 2). In every case the enzyme has
proven to be dimeric, and in most cases two or more loci code for ADH
subunits. The ADH isozyme system has proven especially interesting to
study because the individual loci often exhibit tissue specificity and
can be induced by anaerobic conditions (3). We report here our observa-
tions on the ADH system in pea.
Tissues were examined for ADH expression by crushing small samples
in Tris malate pH 8.5 extraction buffer and subjecting this extract to
starch gel eletrophoresis on Tris/borate buffer system at pH 8.1 (4).
After electrophoresis an anodal slice was stained for ADH activity using
a standard assay: 0.1 M Tris/HCl pH 8.0, 2% ethanol, 0.4 mM NAD, 0.3 mM
MTT and 0.1 mM PMS.
Dry and imbibed seeds express a single major band of ADH activity.
Seeds that have been imbibed and submerged or otherwise placed in an
anaerobic environment exhibit three ADH activity bands. These results
suggest a two-locus isozyme system, one of which is normally expressed
in cotyledon tissue and the other induced by anaerobic conditions. Such
a system has been described in several species including chickpea (5).
ADH activity could not be detected in well aerated root and leaflet
tissue, but a three-banded phenotype was observed in root extracts after
the roots had been immersed in water for 12 or more hours. Developing
testa and cotyledon extracts also gave a three-banded ADH banding
pattern when subjected to electrophoretic analysis. ADH activity can
also be induced in leaf tissue by anaerobic conditions.
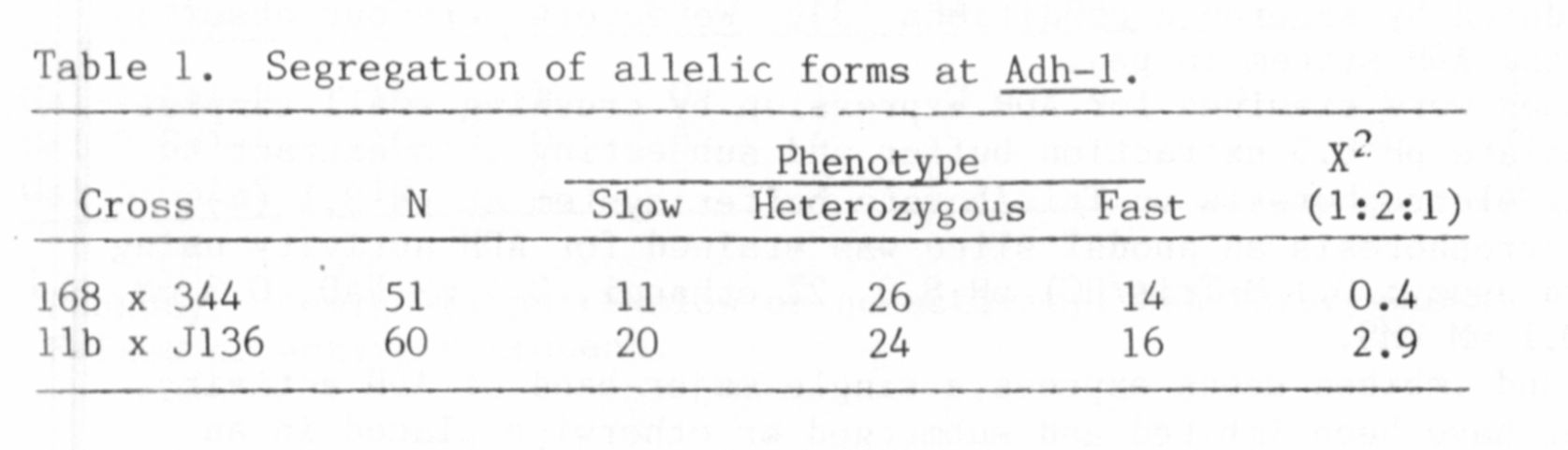
A genetic test of the two-locus model was made possible by the dis-
covery of a relatively rare polymorphism in the ADH phenotype. The
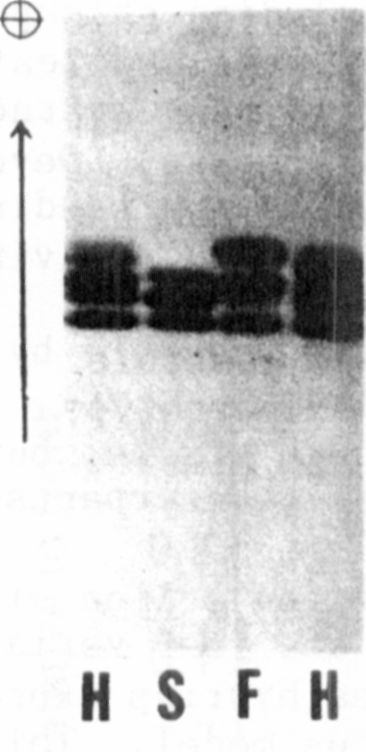
variant lines showed a triplet of bands in anaerobic tissue, but the two
more anodal bands were faster migrating than their counterparts in the
more common phenotype (Fig. 1).
Hybrid plants were produced from a cross between a line with the
normal slow-migrating form and a line exhibiting the fast variant.
Under anaerobic conditions, root tissue from these hybrids expressed the
six-banded ADH phenotype predicted by the two-locus model. This finding
confirmed the heterodimeric nature of the intermediate band in each of
the triplets expressed in the parental lines because a third band was
not formed between these two bands. Self-pollination of the hybrid
plants gave an F2 population which contained both parental ADH pheno-
types as well as that of the hybrid (Table 1). The relative number of
plants expressing each of these phenolypes corresponded to that expected
for segregation of two alleles at a single locus, Adh-1. Most anodal
bands of the parental phenotypes may be designated ADH-laa and ADH-lbb
because they are the homodimeric combinations of the subunits produced
by the a and b alleles of Adh-1. The ADH band observed in the hybrid at
a position intermediate between these homodimeric bands is the intra-
genic heterodimer, consisting of a subunit from the la allele and a sub-
unit from the lb allele. The middle band in each parental ADH triplet
must be an intergenic heterodimer, consisting of a subunit of ADH-1 and
a subunit of ADH-2. In both parents the slowest migrating band is the
homodimer of ADH-2 subunits. Adh-1 is the locus expressed in dry seed
tissue while Adh-2 appears to be induced only under anaerobic condi-
tions .