Schwarz, H. P. - � Institute ol Genetics, University ol Bonn
Federal Republic ol Germany
In algae and higher plants chlorophyll is always associated with
specific thylakoid membrane proteins to form chlorophyll protein com-
plexes. Some of these complexes contain chlorophyll a and b, both being
present in approximately equal amounts. The main function of chloro-
phyll a/b protein complexes is light harvesting; therefore, they are
called light-harvesting chlorophyll a/b protein complexes (LHGP). The
protein moieties are called apoproteins.
By investigating chlorophyll b-deficient mutants it might be possi-
ble to elucidate more details concerning the role of chlorophyll b, its
binding to proteins, and its role in photosynthetic functions. For this
reason two chlorophyll mutants obtained from Dr. Gottscha1k's collection
were analyzed using morphological and biochemical methods.
The mutants exhibit a similar phenotype caused by a total chloro-
phyll reduction of about 65%. One mutant, 130A, lacks chlorophyll b
altogether, whereas the other mutant, chlorotica-29, possesses 24%
chlorophyll b in relation to the initial line, cv 'Dippes Gelbe Vik-
toria'. Besides large differences in the structural development of thy-
lakoid membrane systems, both mutants are characterized by differences
in thylakoid membrane polypeptide composition as demonstrated by poly-
acrylamide gel electrophoresis (1). This polypeptide pattern was rein-
vestigated by two-dimensional gel electrophoresis.
Proteins were isolated from thylakoid membrane fractions by mild
treatment with sodium dodecyl sulfate (SDS), and separated on polyacryl-
amide slab gels using Laemmli's Tris-glycine buffer system. separation
gels of the first dimension (10-20% acylamide concentration) contained
0.1% SDS. Unstained lanes were cut out and polymerized in the stacking
gel (1.5 mm thick) for second dimension gels containing 0.1% SDS and 5M
urea. As a control, protein samples isolated from the different geno-
types were one-dimensional1y electrophoresed on both gel systems with
and without 5M urea. The polypeptide pattern became visible by staining
gels with Coomassie brilliant blue G-230.
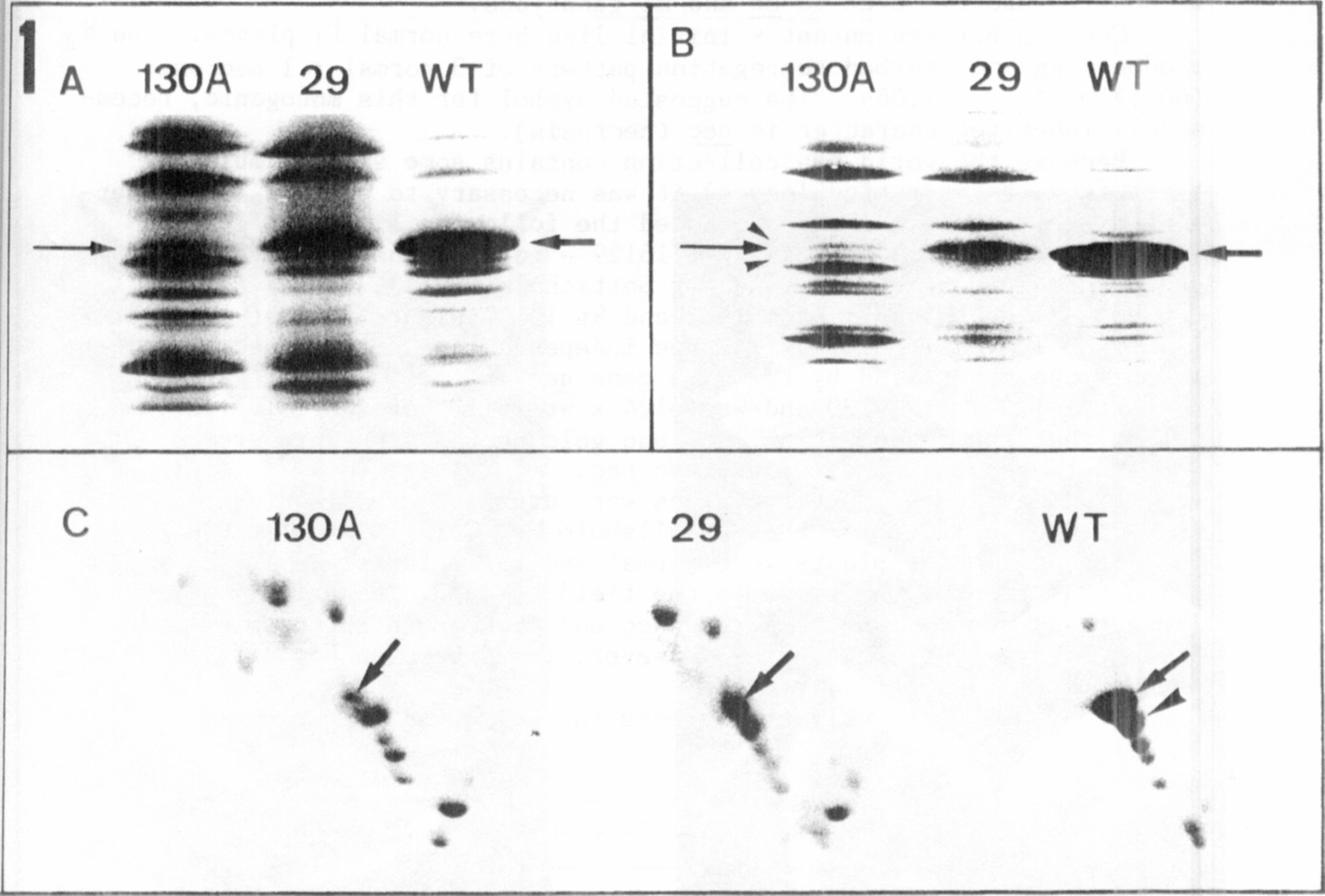
The different electrophoregrams of the thylakoid membnane polypep-
tides isolated from the three genotypes are presented in Fig. 1. The
relative molecular mass range between 20 and 35 kD is shown
because differences are located in this range. In one-dimensional se-
paration of wild type (WT) the apoprotein band of LHCP from photosystem
11 is predominant (arrows, Fig. 1 A, B). This band is drastically re-
duced in chlorotica-29, and can only be detected as a very thin bind in
chlorophyll b-deficient mutant 130A (arrows, Fig. 1, A,B). In the SDS
gel (Fig. IB) two additional faint bands are visible in the lane contai-
ning 130A (arrowheads). In the gel with 5M urea (Fig. 1A) the polypep-
tide pattern is slightly different; resolution is not as good as in the
SDS gel but the reduction of the apoprotein of LHCP is avident in lanes
containing mutants.
Two-dimensional gel electrophoresis (Fig. IC) results in a better
resolution compared with the one-dimensional gels. The reduction of
LHCP apoprotein is well demonstrated (arrows, Fig. 1C). The staining
intensity of. the spots in gels of mutants is more pronounced. This
phenomenon is caused by the marked decrease in apoprotein. Applying the
same protein concentration on gels this reduction alters the relative
concentration of each polypeptide. To identify the spots with lower