GENETICALLY CAUSED DIFFERENCES IN SOLUBLE AUXIN-BINDING: CORRELATION
WITH INTERNODE LENGTH AND CALLUS FORMATION
Jacobsen, H.-J. Institute of Genetics
University of Bonn, Federal Republic of Germany
In tissue culture studies with crop plants, one often is confronted
with recalcitrant regeneration when hybrid lines or cultivars serve as
starting material. In pea, the use of defined mutants resulted in a
certain success of regenerating plants from callus tissues (7, 8) or
somatic embryos from calli transferred to liquid medium (4, 6). These
results indicate that success or failure of in vitro regeneration is
determined in part by the genotype. Since attempts to use the potential
of in vitro technology for crop improvement depend on the regeneration
of intact plants from isolated and selected cells or protoplasts, the
lack of complete understanding and control of this process is a key
problem in most important crops, e.g. large seeded legumes such as pea.
Thus, investigations were carried out in an attempt to correlate the
known genetic control of in vitro behavior with observations on the
molecular level of hormone recognition in plants.
The existence in pea of soluble cytoplasmic auxin-binding sites is
well established (1, 2, 3, 5). Epicotyls of etiolated pea seedlings
exhibit soluble auxin-binding with all characteristics required (or re-
ceptor function of these proteins. These proteins are a) specific for
auxins, b) have a high affinity to bind active auxins, and c) show a
time-dependent occurrence. One binding site (sABP1) is found in the
cytosol of etiolated pea seedlings aged 7 days or more; a second site
(sABP2) can be found in seedlings 9 days after germination (3). These
two binding sites can further be distinguished by their different pi and
their different dissociation constants.
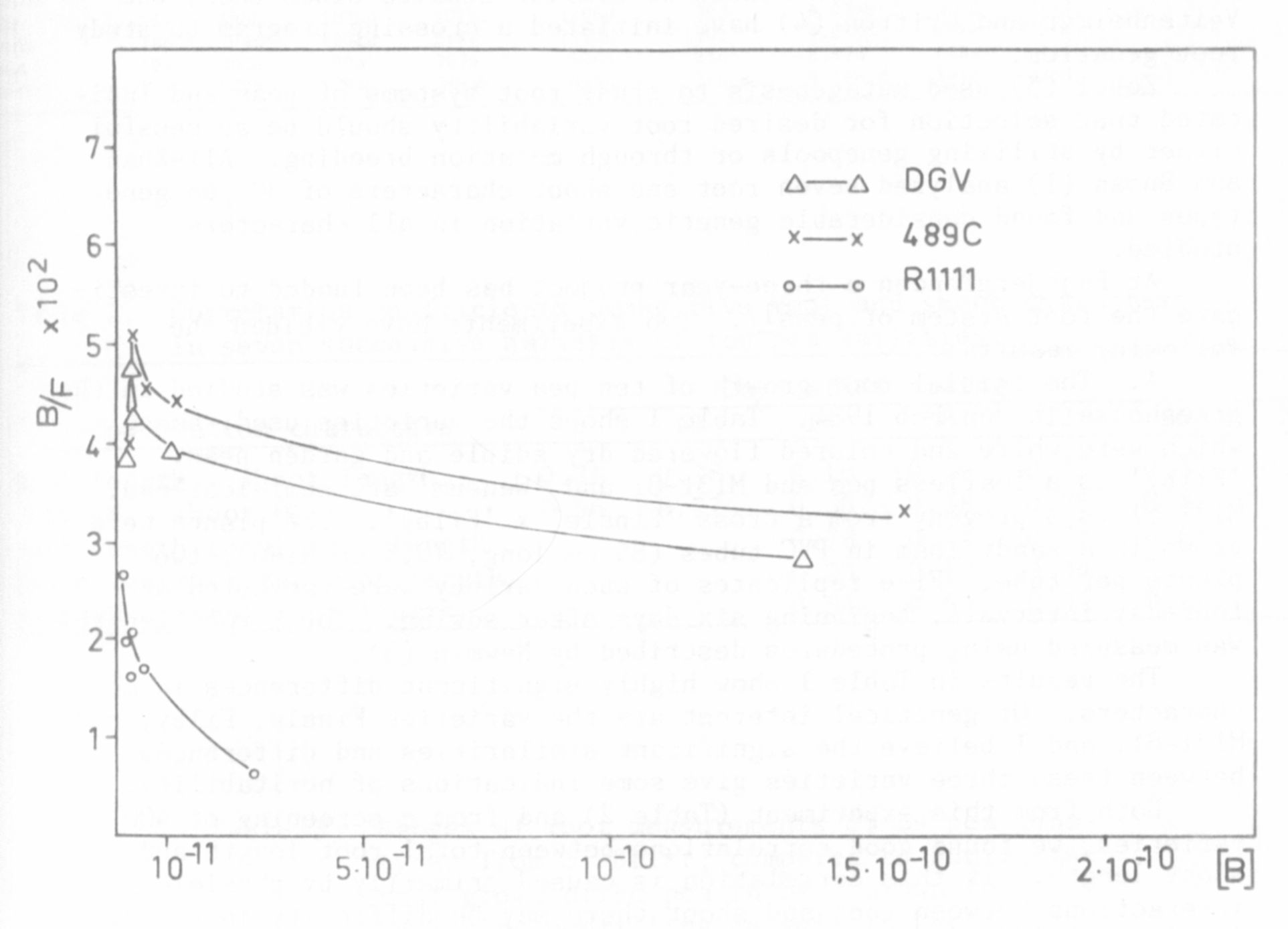
In recent experiments it was demonstrated that in a recombinant
derived from a cross between mutant 489C and the initial line (DGV) the
second binding site was not expressed as in the parent lines (Fig. 1).
Both soluble auxin-binding sites evidently were present in each of the
two parental lines whereas only one was evident in recombinant R 1111.
The internode length of the recombinant is twice that of DGV but the
number of internodes is the same, so the height of the mutant in the
field is about double that of the initial line (Loennig, pers. comm.).
On the other hand, R 1111 reacts best in callus induction experiments.
While calli formed from explants of non-elongated internodes (6 mm) of
DGV and 489C after 3 days were 8.11 +/- 0.8 mm and 8.29 +/- 1.0 mm, the mean
length of these calli in the recombinant was 9.27 +/- 1.2mm. From these
data it is suggested that the different in vitro reaction reflects the
differences observed with the soluble auxin-binding kinetics.