Ohlendorf (14) presented evidence for two partially dominant genes,
A and B, controlling nodulation resistance. The two genes were found in
different lines from Afghanistan; it is not known if they are similar to
Lie's Afghanistan line. In both the F1 and F2 generations of crosses
between completely resistant "Afgh I" and occasionally low (<4) nodulat-
ing "Afgh III", approximately 15% of the plants had higher (4-25) nodule
counts than the parental lines. When either line was crossed with
'Bottnia' (35-102), the F. plants varied from 0-30 nodules and F2
populations exhibited a wide and continuous range of nodulation (0-105)
which was attributed to the segregation of a third gene, C, for nodule
number. If the F2 populations are arbitrarily divided into two nodula-
tion classes, <30:>30, the data fit a dihybrid 13:3 ratio. However,
nodulation patterns of F3 progeny of selected F2 plants did not confirm
the existence of all the expected genotypes.
At the Boyce Thompson Institute, reciprocal crosses between an
inbred line of Lie's Afghanistan (which has no, or rarely, a few (<5)
nodules) and 'Sparkle' (20-90 nodules) yield F1 plants with 5-50 nodules
when grown in vermiculite inoculated with R. leguminosarum 128C53.
Nodule number on F1 plants tends to be lower when Afghanistan is the
female parent. F2 populations show continuous variation for nodulation
with 20-27% nonnods, 10-30% 1-9, 25-50% 10-50, and 10-30% >50 nodules.
The segregation for presence versus absence of nodules fit that expected
for two alleles at a single locus (sym-2). How to delineate classes
within the non-parental intermediates is unclear. We hoped to clarify
the segregation ratios by repeatedly backcrossing selected non-
nodulating plants to 'Sparkle' and eliminating variation due to genes
modifying nodule numbers. With rhizobial strain 128C53, Afghanistan x
Sparkle BC4F1 plants all noduled in the Sparkle parental range. BC4F2
populations still included 20-40% non-parental type intermediate
nodulating plants and 5-25% nonnods. Selection against genes for low
nodule number and for strain specific nodulation resistance will require
testing each plant with 2 strains of R. leguminosarum - one infective,
eg. TOM, to select for high number of nodules and one to which
Afghanistan is nodulation resistant, eg. 128C53. Afghanistan x Sparkle
F2 populations scored with R. leguminosarum strain TOM all nodulated
with a range of 10-90 nodules. Testcrosses of F1 plants to both parents
were made. With strain 128C53, 42% of the progeny from F1's x
Afghanistan and reciprocals were nonnod, while the remaining 58% nodu-
lated in the range of the F1's. Backcrossing F1's to Sparkle yielded
nodulating plants with wide variation in nodule number.
We have investigated the genetics of nodulation resistance by
analyzing for linkage between non-nodulation and isozyme loci which
segregated In F2 progeny of Sparkle x Afghanistan and reciprocal crosses
as well as F plants testcrossed to Afghanistan.
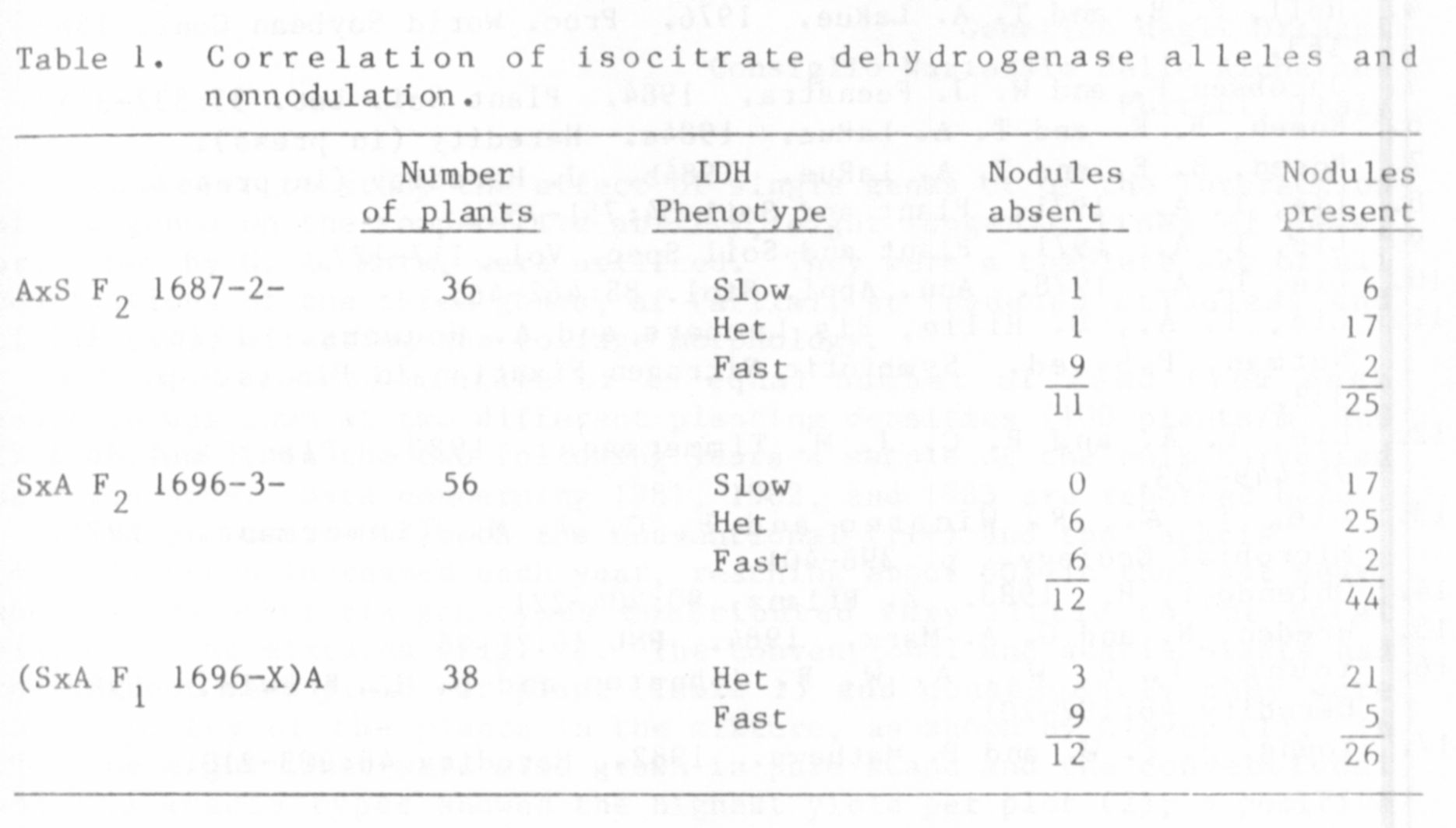
A correlation was observed (Table 1) with the isocitrate
dehydrogenase (IDH) phenotype, which is specified by the locus Idh on
chromosome 1 (15). The Afghanistan parent was homozygous for the "fast"
allele at Idh and Sparkle was homozygous for the "slow" allele. Of the
23 F2 individuals resistant to nodulation, 15 were homozygous for the
"fast' allele at Idh. In the testcross progeny, 9 out of 12 nonnodulat-
ing plants were "fast", a significant deviation from random assortment.
No correlation was observed between low nodule number (1-5) and the IDH